Abstract
Background
Deficits in antisaccade (AS) and smooth pursuit eye movements (SPEM) are promising endophenotypes in genetic studies of schizophrenia. The Icelandic population lends itself ideally to genetic studies due to its ethnic homogeneity and well-documented genealogy. The primary aim of this study was to assess AS and SPEM performance in a large Icelandic sample. Additional aims were to investigate the relationship between AS and SPEM task performance and to assess internal consistency, within-session performance changes and effects of SPEM target velocity on performance.
Method
Patients with schizophrenia (N = 118) and healthy controls (N = 109) matched for age and gender underwent infrared oculographic assessment of AS and SPEM (at target velocities of 12°, 24° and 36°/s).
Results
On the AS task patients displayed significantly more reflexive errors, longer latency, increased intra-individual latency variability, and reduced amplitude gain compared to controls. On the SPEM task, patients had significantly lower velocity gain and more frequent saccades during pursuit at all velocities, but group differences in velocity gain increased with increasing target velocity. Internal consistency of performance was high for all variables in both groups (Cronbach’s alpha >0.77 for AS and >0.85 for SPEM) except for AS spatial error in patients (alpha = 0.38). A moderate association was found between AS and SPEM performance. By and large, patients and controls showed similar patterns of systematic within-session performance changes.
Conclusions
Our findings confirm the existence of robust eye movement deficits in schizophrenia in a large sample. These measures may be studied as endophenotypes in future studies of potential schizophrenia risk genotypes in the genetically homogenous Icelandic population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is strong evidence for involvement of genetic factors in the etiology of schizophrenia. However, the identification of causative genes is complicated by the considerable variability of symptoms and disease course between individuals with schizophrenia. Studying endophenotypes, i.e., biological or behavioral features thought to be more direct expressions of disease related genes than the clinical phenotype, may circumvent this problem and facilitate genetic studies of schizophrenia [3, 51].
Impairments on antisaccade (AS) [11, 17, 19, 39] and smooth pursuit eye movements (SPEM) [30, 33, 49, 50] are well-studied schizophrenia endophenotypes. The AS task requires the subject to generate a rapid eye movement (saccade) to a location opposite of a peripheral visual stimulus. All studies of AS eye movements in schizophrenia have demonstrated more frequent reflexive errors (glances towards the target) in patients than controls [35]. Most studies have found AS latency (time interval between target movement and eye movement) to be prolonged in schizophrenia [37, 44] and there is evidence for decreased AS amplitude gain [17, 19, 37]. In the SPEM task the subject follows a slowly moving visual stimulus. Schizophrenia patients have lower velocity gain and more frequent saccades than healthy individuals [50].
Support for the validity of AS and SPEM endophenotypes comes from studies showing that healthy relatives of schizophrenia patients perform worse than subjects with no family history of psychosis [11, 28]. Furthermore, performance is stable over time [7, 18] and twin studies indicate that AS and SPEM performance is considerably heritable [40, 54]. Taken together, these findings suggest that SPEM and AS deficits are promising schizophrenia endophenotypes. Additionally, research using oculomotor tasks has contributed to our understanding of the impairments in cognitive functions in schizophrenia and their pathophysiology [4, 58].
The present study aimed to add to this body of evidence in three ways. First, we aimed to replicate the observation of performance deficits in schizophrenia by studying a large and genetically homogenous Icelandic sample. Second, we explored interrelationships between AS and SPEM variables to determine whether these endophenotypes index overlapping or separate genetic factors. Third, we wished to investigate performance characteristics such as intra-individual variability, internal consistency, within-session changes and SPEM target velocity.
Concerning interrelations between AS and SPEM, neuropsychological and brain imaging studies indicate that these deficits may be based on prefrontal cortex dysfunction [6]. Studies investigating correlations between AS and SPEM task performance have provided inconsistent findings. While some have found an association [56, 68, 70] others have not [34, 62]. This issue is of importance, because a correlation would raise the possibility that the deficits may be related to common vulnerability genes for schizophrenia. This question is best studied in large samples in order to avoid failures to detect small correlations due to low power.
Internal consistency is an important reliability marker and provides a measure of the stability of task performance within one session. This measure has to our knowledge been systematically addressed in only two previous studies of AS and SPEM. For SPEM, high internal consistency was found in healthy [18] and schizophrenia subjects [8]. For AS, a study showed high consistency in healthy individuals [18]; however, this has not been studied in schizophrenia.
Relatedly, relatively few neurocognitive and psychophysiological studies have addressed systematic within-session performance changes (e.g., improvement or deterioration within one session) or intra-individual performance variability (e.g., dispersion) in schizophrenia. By reporting the mean performance of a testing session that involves a series of trials it is possible that some important abnormal characteristics related to within-session performance changes or variability are missed. The importance of studying intra-individual performance variability in psychiatric populations has been demonstrated in recent neuropsychological studies [36], where variability measures provided considerably larger group effect sizes [45] and were better able to distinguish clinical groups [2] than the mean or median. Regarding oculomotor tasks, previous studies on healthy subjects have shown some within-session performance changes, which have been related to factors such as learning (improvement) and fatigue or boredom (deterioration) [18, 73]. Systematic within-session changes in AS and SPEM and intra-individual variability on saccadic tasks have to our knowledge not been studied in schizophrenia.
A final issue that will be addressed here concerns effects of target velocity on SPEM performance in schizophrenia. Schizophrenia patients and their relatives show impaired motion perception, which correlates with SPEM performance [10]. It has been suggested that deficits in processing internal representations of target motion contribute to abnormal SPEM [79]. At faster target velocities it becomes more difficult to correct retinal mismatch between target velocity and eye velocity. This correction probably depends on a corrective feedback loop, where an internal representation (extraretinal signal) of target motion activates the pursuit system [47]. Furthermore, recent functional magnetic resonance imaging (fMRI) studies have shown reduced activity in motion processing pathways in extrastriate [48] and medial superior temporal cortex, frontal eye field, supplemental eye field and anterior cingulate during SPEM in schizophrenia [31]. In the current study SPEM performance was assessed at three different target velocities in order to progressively tax the pursuit system.
This study represents the largest single-centre case-control study of AS and SPEM in schizophrenia and may thus provide important information on the reliability of eye movement measures and whether and how effects of time and target velocity on performance may differ between patients and controls. From a genetic point of view, there are several advantages of conducting this research in Iceland. First, the nation was an isolated island population up until the late twentieth century and is therefore relatively genetically homogenous [27]. Secondly, there is a reliable genealogy database containing records on approximately half of the adult population ever living in Iceland (716,000 individuals). Finally, access to population and medical records is excellent.
We hypothesized that schizophrenia patients would perform worse than healthy subjects on AS and SPEM. We expected at best moderate relationships between AS and SPEM performance. We also explored within-session performance changes and we expected greater group differences in SPEM with increasing target velocity.
Method
Subjects
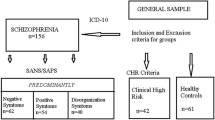
Demographic and clinical data are summarized in Table 1. Schizophrenia patients were recruited from the Division of Psychiatry at Landspitali-University Hospital in Reykjavik. This is the main psychiatric center in the country, where over 90% of all schizophrenia patients in Iceland are treated. The treating psychiatrists introduced the study to patients and obtained permission for researchers to contact them. Of 272 patients approached, 78 were excluded because they were too ill or because of significant co-morbidity. Seventy-four patients declined participation. One patient withdrew consent and one patient was excluded due to being of non-Icelandic descent. Data were collected from the remaining 118 patients. Diagnoses were confirmed by experienced psychiatrists according to Research Diagnostic Criteria (RDC) [75] using the Schedule of Affective Disorders and Schizophrenia—Lifetime Version (SADS-L) [74].
Over 90% of patients were taking antipsychotic medications (14.0% typical, 51.3% atypical and 28.7% both typical and atypical). Approximately a third (28.7%) was on clozapine. The majority of patients were smokers (73.9%). Patients’ current symptom levels were assessed using the positive and negative syndrome scale (PANSS) [41].
Healthy controls (N = 113) were recruited from the local community. They were screened for history of axis I psychiatric disorders using the MINI Neuropsychiatric Interview [71]. Those who had first or second-degree relatives with schizophrenia or other psychotic disorder were excluded. Four controls were excluded because they had second-degree relatives with schizophrenia leaving 109 subjects for eye movement testing. Twenty-one percent of the controls were smokers.
Subjects with a history of neurological illness (e.g., stroke, seizures, Parkinson’s disease), eye abnormalities, head injury (causing loss of consciousness), and substance abuse/dependence in the past 12 months were excluded. All participants were Icelandic, between 18 and 55 years of age and provided written, informed consent. The Icelandic Scientific Ethics Committee approved the study.
Eye movements
Eye movements were recorded using infrared oculography (IRIS 6500; Skalar Medical BV, Delft, The Netherlands). Recordings were taken from the left eye and sampled at 500 Hz. Participants were seated in a chair 57 cm from a 17-in. monitor. Head movements were minimized using a chinrest. A white circular target (0.3°) was presented on a black background. Tasks were administered in the following order: prosaccade, AS, fixation and SPEM. Each task was preceded by a 3-point calibration trial (0°, ±12°).
Each prosaccade trial began with the target in the central location (0°) for a random duration of 1,000–2,000 ms. The target then stepped to one of four peripheral locations (±6°, ±12°) where it remained for 1,000 ms. Each peripheral location was used 15 times, resulting in a total of 60 trials presented in random order. Four practice trials were carried out. Participants were instructed to keep their eyes on the target and follow it as accurately as possible.
In the AS task, the target movements were identical to those in the prosaccade task. However, on this occasion participants were instructed to look at the target while in the central position and redirect their gaze to the exact mirror image location of the target as soon as it moved to the side.
In the fixation task the target remained stationary in each of three locations for 20 s each. The first target location was randomly right or left (±12°), the second location was central (0°), the third location was the peripheral location that had not been used in the first trial (±12°), and the final location was again central (0°). Participants were instructed to focus their gaze on the target as accurately as possible.
For SPEM, a triangular target waveform was employed three times at 12°, 24° and 36°/s, respectively. The target appeared in the central position (0°) and then moved horizontally to ±12°, where it reversed abruptly and moved to the opposite side. The direction of the first ramp was random (right or left). The first ramp (from 0° to ±12°) was considered practice and was not included in the analyses. A total of 16.5 half-cycles were run at each target velocity. Participants were instructed to keep their eyes on the target as closely as possible.
For AS, prosaccade and fixation data analysis, Eyemap 2.1 (AMTech GmbH, Weinheim, Germany) was used. SPEM analysis was carried out using purpose-written routines in LabView. All data were scored blind to group status by one rater (MH) and confirmed by a second rater (UE). Inter- and intra-rater reliabilities were high (r = 0.95–0.99).
For AS it was not possible to analyze data from ten patients and four controls due to poor quality. This was either because of difficulties the subjects had in performing the task or due to excessive head movements and eye blinking during the task. Saccades were automatically detected on minimum amplitude (1°), velocity (30°/s) and latency (100 ms) criteria. The rater individually categorized saccades that had been identified by the software.
A correct AS trial occurred when the participant performed a primary saccade in the direction opposite to the peripheral target. A reflexive error was counted when the participant performed a primary saccade towards the peripheral target. A corrective saccade was counted when an error was followed by a saccade in the opposite direction.
A number of dependent variables were derived. The reflexive error rate reflects the percentage of error trials over the total number of valid trials. Latency of correct AS was defined as the time (ms) from target appearance to saccade initiation. AS amplitude gain was calculated as the primary saccade amplitude divided by target amplitude multiplied by 100. Spatial accuracy of correct AS was assessed using the measure of spatial error. Spatial error was obtained by calculating, for each saccade, the percentage of residual error. This is obtained by subtracting the target amplitude from the saccade amplitude and dividing the result by the target amplitude calculated residual error. The absolute value of this term reflects the residual error and is then averaged across all saccades and multiplied by 100. A perfectly accurate saccade thus attracts a spatial error score of 0% and higher scores denote greater spatial error. The percentage of corrective saccades (over the total number of error trials) was also established. For latency, amplitude gain, and spatial error the individual standard deviation was calculated as a measure of intra-individual performance variability.
Finally, these dependent variables were calculated for the total 60 trials as well as for four sub-segments of equal length including approximately 15 trials each. This was done in order to address systematic within-session performance changes.
Prosaccade data from five patients and one control were not used due to poor quality. Prosaccade latency (ms), amplitude gain (%) and spatial error (%), were calculated based on the same criteria as in the AS task. Incorrect prosaccades (subject not following target) were counted. As for the AS task the means and individual standard deviations of these dependent variables were determined for the 60 trials and also for each of four sub-segments of equal length.
SPEM data from two patients and one control for velocity gain and six patients for saccade frequency were not analyzed due to poor quality. Pursuit velocity gain data from one control was not correctly obtained. Velocity gain was calculated by dividing mean eye velocity by target velocity. This was done for sections of pursuit (excluding saccades and eye blinks) during the central half of each ramp excluding the first and last quarters to avoid pursuit initiation and slowing at target turnarounds. These scores were time-weighted and subsequently averaged. Saccades that occurred during SPEM were automatically identified on the basis of minimum amplitude (1°) and velocity (30°/s). The number of saccades was established at each target velocity and divided by the duration of pursuit at each target velocity to yield measures of saccadic frequency (N/s). Velocity gain and saccade frequency was also calculated for four sub-segments of equal length (approximately four half-cycles each).
For fixation, three patients and one control had poor quality data, which were not analyzed. The frequency of saccades during fixation (N/s) was calculated based on the previously described criteria of minimum amplitude and velocity. Saccade frequency was also calculated for each of the four equally long sub-segments representing the two peripheral and two central target locations.
Statistical analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) version 11 (SPSS Inc. Chicago, IL, USA). Level of significance was set to P < 0.05. Outliers were identified using box plots and all extreme values (more than 3 box-lengths from edge of box) were removed. Distributions of oculomotor variables were assessed for normality using the skewness index. If positively (>1) or negatively (<1) skewed, variables were transformed using square root or square transformations, respectively.
Group differences on oculomotor variables were investigated using analysis of variance (ANOVA) with each oculomotor variable as dependent variable and Group (patients vs. controls) as independent variable.
Relationship between AS and SPEM variables was evaluated using Pearson correlation. Correlations were first carried out in the combined sample (patients, controls) and then in each group separately.
Internal consistency of task performance was measured using Cronbach’s coefficient alpha [14]. Cronbach’s coefficient alpha is an index of the average correlation among all items of a psychometric instrument. The alpha coefficient ranges from 0 to 1 and the higher the score, the more reliable the instrument is. The coefficient was calculated using sub-scores of eye movement recordings from the four equally long consecutive sub-segments of each task.
Within-session performance changes were investigated using repeated measures ANOVA for each eye movement variable with TaskDuration (four time segments described above) as within-subject factor and Group (patients, controls) as between-subject factor. Mauchly’s test was considered for each ANOVA to evaluate assumptions of sphericity. If assumptions of sphericity were violated, Greenhouse–Geisser epsilon corrections of degrees of freedom were used.
Repeated measures ANOVA was used for evaluating effects of target velocity on SPEM and Velocity-by-Group interactions with velocity (12°, 24° and 36°/s) as within-subjects factor and Group (patients, controls) as between subjects factor.
Relationships between oculomotor variables and PANSS scores, age of illness onset and illness duration were analyzed using Pearson correlation. Effects of smoking and type of antipsychotic medication on eye movement variables were compared using ANOVA with Smoking Status (smokers, non-smokers) and Antipsychotic (typical, atypical, both, none) as independent variables.
Results
Data from 118 patients (mean age = 41.2, standard deviation = 9.80, 73.7% male) and 109 controls (mean age = 40.8, standard deviation = 9.10, 62.4% male) were analyzed. The groups were matched on sex (χ2 = 2.61; df = 1; P = 0.11) and age (t = 0.30; df = 221; P = 0.77) (Table 1). Descriptive statistics and effect sizes for oculomotor variables are shown in Table 2. Extreme values (outliers) were removed from the dataset for prosaccade: two patients and two controls, AS: two patients and two controls, SPEM: one patient and four controls and fixation: ten patients and three controls.
The following variables were skewed (skewness index): AS latency standard deviation (SD) (1.49), AS spatial error SD (1.64), AS correction (−3.05), prosaccade spatial error (1.30), prosaccade spatial error SD (1.24), SPEM 36°/s saccade frequency (1.44) and fixation saccade frequency (1.92). Inferential statistical analyses were done on transformed variables. Table 2 shows the untransformed data.
Prosaccade
The average rate of incorrect prosaccades (subject not following target) was only 0.47% (SD = 1.32) for patients and 0.22% (SD = 0.79) for controls showing that subjects were generally able and willing to perform the task.
There were significant between-group differences for prosaccade latency (F[1,214] = 9.94; P = 0.002) and spatial error (F[1,214] = 9.86; P = 0.002). Patients had significantly longer saccade latency and greater spatial error than controls. There were also significant group differences in the intra-individual variability (SD) of prosaccade amplitude gain (F[1,214] = 11.38; P = 0.001), latency (F1,214] = 23.89; P < 0.001) and spatial error (F[1,214] = 12.61; P < 0.001). Patients had larger individual performance variability on these variables. No significant difference was seen for prosaccade amplitude gain (F[1,214] = 1.41; P = 0.27).
Internal consistency of performance was high for all variables in both patients and controls (all Cronbach’s alpha > 0.85).
There was a significant TaskDuration effect on prosaccade amplitude gain (F[3,191] = 17.05; P < 0.001) but no TaskDuration-by-Group interaction (P = 0.21). The effect was linear with gain scores decreasing (i.e., becoming more hypometric) over the 4 time segments (P < 0.001).
There was a TaskDuration effect (F[3,191] = 4.88; P = 0.002) and a TaskDuration-by-Group interaction (F[3,191] = 2.77; P = 0.04) on prosaccade latency. The TaskDuration effect was a quadratic effect with latency decreasing after the first quarter and then increasing (P = 0.004). There was also a cubic effect (P = 0.01) with latency decreasing again in the last quarter in patients. The interaction was due to a general decrease in latency over time in controls (P = 0.01) whereas in patients latency increased in the last quarter.
There was a significant TaskDuration effect on prosaccade spatial error (F[3,191] = 11.43; P < 0.001) but no TaskDuration-by-Group interaction (F[3,191] =0.22; P = 0.84). A linear increase in spatial error in the third and fourth quarters was found in both groups (P < 0.001) and also a small cubic effect with an initial small decrease in the second quarter and then a steep increase (P = 0.02). There were no significant TaskDuration effects on the variability of prosaccade latency, amplitude gain or spatial error (all P > 0.05).
Antisaccade
The average rate of corrections (initiation of an AS after an error) was 97.8% (SD = 5.5) for controls and 91.5% (SD = 11.5) for patients.
Significant group differences were present for reflexive error rate (F[1,208] = 106.91; P < 0.001), amplitude gain (F[1,208] = 8.55; P = 0.004), latency (F[1,208 = 19.76; P < 0.001) and latency SD (F[1,208] =20.49; P < 0.001). Patients displayed significantly more reflexive errors, reduced (i.e., hypometric) amplitude gain, and longer and more variable latency than controls. Spatial error (F[1,208] = 3.58; P = 0.06), spatial error SD (F[1,208 = 0.032; P = 0.86), and amplitude gain SD (F[1,208] = 0.01; P = 0.92) did not differ significantly between groups.
Internal consistency was high for all AS variables in both patients and controls (>0.77 and >0.80, respectively) except for spatial error in patients (0.38).
There was a significant TaskDuration effect on reflexive error rate (F[3,198] = 12.09; P < 0.001) but no TaskDuration-by-Group interaction (F[3,198] = 1.01; P = 0.39). The effect was quadratic with error rates decreasing in the second quarter and then increasing in the third and fourth quarters (P < 0.001) (Fig. 1). A significant TaskDuration effect was found on AS amplitude gain (F[3,175] = 5.57; P = 0.001) but no TaskDuration-by-Group interaction (F[3,175] = 1.49; P = 0.22). The effect was mainly linear with gain decreasing with time (P = 0.006). There was also a quadratic effect (P = 0.02) with an increase in gain in the fourth quarter in patients. A TaskDuration effect was found on AS latency (F[3,171] = 2.73; P = 0.049) and there was also a TaskDuration-by-Group interaction (F[3,171] = 2.86; P = 0.04). The main effect was linear with latency increasing with time (P = 0.04). The interaction was due to a cubic effect in patients with latency increasing in the second quarter, decreasing in the third and finally increasing again in the fourth quarter (P = 0.01). There were no significant TaskDuration effects on spatial error (F[3,175] = 1.32; P = 0.27) or the variability of gain (F[3,169] = 1.88; P = 0.14), latency (F[3,169] = 0.34; P = 0.79) and spatial error (F[3,169] = 1.49; P = 0.22).
Smooth pursuit
A repeated measures ANOVA showed a significant main effect of Velocity on SPEM gain (F[2,444 ] = 291.32; P < 0.001), a significant effect of Group (F[1,222] = 29.64; P < 0.001), and a significant Velocity-by-Group interaction (F[2,444] = 14.69; P < 0.001). These effects indicate that gain deteriorated with velocity, patients had lower pursuit gain than controls and group differences increased with increasing target velocity (Fig. 2).
For SPEM saccade frequency there was a significant effect of Velocity (F[2,430] = 422.56; P < 0.001) a significant effect of Group (F[1,220] = 23.77; P < 0.001), but no significant Velocity-by-Group interaction (F[2,440] = 0.27; P = 0.74). This result shows that saccade frequency increased with target velocity and that patients made more saccades than controls but the group difference was not affected by target velocity.
Internal consistency of performance was high for all variables in both groups (>0.85).
Analysing effects of TaskDuration on SPEM, no significant effect was found on velocity gain at 12°/s (F[3,222] = 2.15; P = 0.11) but there was a significant effect at 24°/s (F[3,223] = 3.70; P = 0.015) and 36°/s (F[3,224] = 5.18; P = 0.002). The effect at 24°/s was quadratic with velocity gain increasing in the second quarter and then decreasing (P = 0.02). At 36°/s the effect was mainly cubic with velocity gain increasing in the second quarter, decreasing in the third and finally increasing in controls in the fourth quarter (P = 0.001). There was also an overall linear decrease with time (P = 0.02). There was a TaskDuration-by-Group interaction only at 36°/s (F[3,224] = 3.51; P = 0.02). This interaction was both quadratic (P = 0.03) and linear (P = 0.04) due to an increase in velocity gain in the second quarter and a decrease in the third and fourth quarters in patients.
A significant TaskDuration effect was found on saccade frequency during SPEM at all three target velocities (all P < 0.001) but no TaskDuration-by-Group interactions (all P > 0.05). The TaskDuration effect was linear at all three velocities with saccade frequency decreasing over the duration of the task (all P < 0.001). There was also a quadratic effect with an increase in saccade frequency in the fourth quarter in controls at 12°/s ( P = 0.002) and 24°/s ( P < 0.001) and a small cubic effect at 12°/s ( P = 0.03) due to an increase in the third quarter in patients.
Fixation
Patients made significantly more saccades during fixation than controls (F[1,209] = 17.72, P < 0.001). Internal consistency of performance was high for both patients and controls (>0.85). No significant TaskDuration effect was found on saccade frequency (F[3,225] = 0.45; P = 0.66).
Relationship between AS and SPEM
There were a number of moderate but statistically significant correlations between AS and SPEM measures in the combined sample. Higher AS reflexive errors were associated with lower SPEM velocity gain at 24°/s (r = −0.27; P < 0.001) and 36°/s (r = −0.34; P < 0.001). The error rate was also associated with increased SPEM saccade frequency at all target velocities (all r > 0.23 and P < 0.001). Increased AS latency was associated with decreased SPEM velocity gain at all target velocities (all r < −0.25 and P < 0.001) and higher saccade frequency at 12°/s (r = −0.26; P < 0.001) and 24°/s (r = −0.13; P = 0.03). AS spatial error correlated with SPEM velocity gain at 36°/s (r = −0.15; P = 0.04) and saccade frequency at 12°/s (r = 0.15; P = 0.03) indicating that less accurate AS performance was associated with lower velocity gain and increased saccade frequency during SPEM. There were no significant correlations between AS amplitude gain and SPEM variables.
In patients, longer AS latency was associated with decreased SPEM velocity gain at all target velocities (all r < −0.22; all P < 0.02). No other correlations were significant.
In controls, increased AS reflexive error rate was associated with lower SPEM velocity gain at 36°/s (r = −0.22; P = 0.028) and with higher saccade frequency at 24°/s (r = 0.20; P = 0.047) and 36°/s (r = 0.20; P = 0.038). Longer AS latency was associated with lower SPEM velocity gain at all three target velocities (all r < −0.20; all P < 0.05) and with higher saccade frequency at 12°/s (r = 0.27; P = 0.006).
Relationships with clinical variables
Patients’ age at onset of illness did not correlate with any eye movement variable (all P > 0.05). While controlling for age, duration of illness correlated with AS correction rate (r = −0.27; P = 0.008), SPEM velocity gain at 24°/s (r = −0.36; P < 0.001) and 36°/s (r = −0.34; P < 0.001) and number of fixation saccades (r = 0.23; P = 0.02). Longer duration of illness was associated with lower AS correction rate (see Fig. 3), lower SPEM gain and a larger number of fixation saccades.
AS correction rate was the only eye movement variable, which correlated significantly with PANSS scores (negative scale score; r = −0.29, P = 0.005, and total score; r = −0.24, P = 0.02); patients with higher negative and total PANSS scores had a lower correction rate (see Fig. 3).
ANOVA of patients taking different antipsychotic medications (typical, atypical, both, none) did not show any significant effects of medication type on eye movements (all P > 0.12). Due to the unique pharmacology of clozapine patients taking the drug were compared to those who were not. No significant effect of clozapine was found (all P > 0.07). There were no effects of smoking (all P > 0.1).
Discussion
This is a study of eye movements in a large sample of schizophrenia patients and healthy controls belonging to the genetically homogenous population of Iceland. It is the largest single centre case-control study examining both AS and SPEM. The main purpose was to confirm and validate the well-known observation of eye movement deficits in schizophrenia. Additionally, we examined the relationship between AS and SPEM and investigated measures of systematic and random performance variability. The next step of this work is to study in this unique Icelandic sample the relationship between eye movement endophenotypes and recently described schizophrenia risk genotypes [26, 67].
On the AS task patients were found to have significantly higher error rate, lower amplitude gain and longer and more variable latency than healthy subjects. During SPEM, velocity gain was significantly lower and saccade frequency higher. The largest effect size in the study was observed for reflexive errors in the AS task.
This present study replicates all previous studies of AS in schizophrenia, which have found increased rates of reflexive error rates in schizophrenia compared to healthy controls [5, 35]. The study strongly supports that schizophrenia patients have difficulty inhibiting reflexive saccades during the AS task, which may be related to lack of inhibitory control in prefrontal cortex [58] or an impaired ability to appropriately activate a volitional AS response [66]. The finding of longer latency of correct AS in patients than healthy controls, replicates what a number of previous studies have demonstrated [23, 37, 44] and suggests a deficit in volitional response generation [65]. Additionally, patients had increased standard deviations of AS latencies, in agreement with recent evidence of increased intra-individual variability in schizophrenia [36]. Interestingly, however, variability of spatial accuracy measures (AS amplitude gain SD and AS spatial error SD) was not significantly increased compared to controls, suggesting that the deficit in intra-individual performance variability on the AS task in schizophrenia may be specific to temporal but not spatial variability of responses.
Several previous studies have found decreased AS amplitude gain in patients with schizophrenia [37, 59]. The ability to match saccade amplitude to target amplitude depends on sensorimotor processes involved in transforming the covertly encoded visual target location into a motor output, a process that requires a shift in signal processing from intraparietal sulcus to frontal eye field [55]. Although patients in the present study were found to have significantly lower amplitude gain than controls the difference was due to the fact that the controls had a mean gain of −107% (overshooting) and the patients had a mean gain of −93% (undershooting). The mean gain scores of the two groups were therefore equally far (in opposite directions) from the perfect gain score of −100%. The measure of spatial error, which provides a spatial accuracy score independent of hypo- or hypermetria, consequently did not differ between groups. It is unclear what the primary AS spatial accuracy deficit in schizophrenia is; however, the present data in conjunction with previous studies [17, 19, 29, 34, 37] suggest that volitional saccades are most commonly of smaller amplitudes compared to controls.
Concerning the prosaccade task, patients had significantly longer latency, higher spatial error and more variable amplitude gain, latency and spatial error than controls. Previous studies of prosaccade eye movements in schizophrenia have provided inconsistent findings. Most studies have found no significant differences in prosaccade latency and spatial accuracy between patients and controls, suggesting that basic saccadic mechanisms are intact in schizophrenia [11, 13, 37, 46, 76]. However, other studies have demonstrated prolonged latency [20, 53] and reduced accuracy [9, 69] in patients.
Patients also made significantly more saccades during fixation than controls. Previous studies of visual fixation in schizophrenia have obtained conflicting results. While some studies have found fixation to be normal [24, 43], others have not [1, 15]. The contradictory findings of prosaccade and fixation studies in schizophrenia may possibly be attributed to heterogeneity in subject populations and methods of data analysis between studies.
On the SPEM task patients had significantly lower velocity gain and higher saccade frequency than controls at all three target velocities, replicating what most studies using similar methods have found [22, 34, 63, 78]. Recent fMRI studies have shown that schizophrenia patients have decreased activity in extraretinal motion processing pathways during SPEM [31] and also compensatory activity increase in the prefronto-thalamo-cerebellar circuit [61].
Although both AS and SPEM deficits have been proposed as endophenotypes in schizophrenia and both have been related to frontal brain dysfunctions [6] it has not been established whether they reflect the same risk genes. Only a few studies have investigated the association between AS and SPEM measures and results have been inconsistent. While some have found higher reflexive error rate on the AS task to correlate with worse performance on the SPEM task [56, 68, 70] others have not [34, 62]. In the present study only moderate associations were found between the two tasks. Associations that were observed in the combined group were not always significant in each group. We would like to argue therefore that the two measures are likely to tap largely separate sources of genetic risk.
All oculomotor variables displayed very good internal consistency, except AS spatial error in patients. This finding is similar to what has previously been reported in studies of AS and SPEM in healthy subjects [18] and SPEM in schizophrenia patients [8]. The high internal consistency of these measures indicates that inter-individual differences in performance are consistent throughout the session and that these can be reliably measured.
Some systematic within-session changes in performance were observed in both patients and controls. The AS reflexive error rate first decreased and then increased in both groups, which may indicate that it took subjects some time to adapt to the task and improve, but towards the end fatigue was starting to have an effect. A similar effect of time on reflexive error rate was found in a study of healthy males [73]. The present study extends these findings by showing that this effect is also present in schizophrenia patients, thus representing a process that may be unimpaired in schizophrenia.
AS amplitudes became smaller over time in both groups, indicating that AS hypometria might be related to task duration. This suggests that longer task duration may increase demands on sensory-motor pathways involved in processing the target location information into a motor response. Effects on saccade amplitudes were previously described [60]. Mosimann et al. found that making saccades volitional through instructions led to hypometric amplitudes. Together, this pattern suggests that an increased volitional component (or effort) may be related to saccade hypometria; whether these factors account for the hypometric antisaccade amplitudes in schizophrenia patients and their relatives [17, 19] remains to be investigated further.
There was a moderate increase in AS latency with time in both groups. This increase in reaction time may have been related to fatigue.
Similar to AS, prosaccade amplitudes decreased with time. The TaskDuration effect on prosaccade latency was quadratic with a shorter reaction time in the second segment, which may have been due to effects of practice, followed by a slower response possible because of fatigue. There was a TaskDuration-by-Group interaction due to a linear decrease in latency in controls indicating a stronger improvement effect in controls.
SPEM velocity gain generally decreased with time. There was an interaction effect at the fastest velocity because of an increase in gain in the second quarter but then a linear decline in patients. This effect may be ascribed to increasing fatigue or boredom with time, which affected patients more than controls. Saccade frequency decreased with time in both groups, which may be a fast learning effect allowing subjects to optimally stabilize their gaze on the target. A comparable reduction in catch-up saccades was found in healthy subjects in a study by Ettinger et al. [18].
Overall, therefore, some within-session performance changes were found in both patients and controls, which are most likely related to effects of practice and fatigue. These changes were for the most part similar in patients and controls and the only variables showing TaskDuration-by-Group interactions were prosaccade and AS latency and SPEM gain at the fastest target velocity; for these variables patients displayed greater variability in performance over time than controls.
SPEM performance deteriorated with increasing target velocity in both groups and for velocity gain this effect was significantly stronger in patients than controls. A similar interaction was found in a previous study comparing velocity gain in chronic schizophrenia patients, first episode patients and healthy controls at four target velocities (10, 20, 30 and 36°/s) [32]. The present findings therefore support previous suggestions of a motion processing deficit in schizophrenia [10] and add to the evidence that the difference in SPEM velocity gain between patients and controls increases with increasing target velocity.
In the present study only AS correction rate correlated weakly with symptom scores. Higher total and negative scale scores were associated with lower correction rate. An association between negative symptoms and performance, on both AS [12, 80] and SPEM [38, 57, 77] was demonstrated previously. However, there are also several studies, which have not found this association [21, 25, 37, 64].
Patients’ age at illness onset did not correlate with eye movement performance. However, longer duration of illness was associated with lower correction rate on the AS task, decreased SPEM velocity gain and increased rate of saccades during fixation. Although most previous studies have not found a significant association between SPEM and duration of illness or number of hospitalizations [21, 38, 42], there are studies which have found worse SPEM with higher age at onset of psychosis [72, 77] and longer duration of illness [52]. No relationship was found between AS and SPEM performance and type of antipsychotic medication.
The only eye movement measure which was associated with more than one clinical variable was the AS correction rate. The fact that there were few and only weak associations between clinical variables and other measures of eye movement performance suggests that these oculomotor deficits are likely to represent relatively state-independent endophenotypic markers of schizophrenia.
No effects of smoking on eye movements were observed in this study. Several recent studies have shown that nicotine use improves performance on several neurocognitive tasks including AS and SPEM [16]. In those studies nicotine administration was experimentally manipulated and eye movements were measured within minutes of nicotine use and following abstinence.
In conclusion, this study confirms the existence of significant deficits in AS and SPEM in schizophrenia in a large sample. Only moderate relationships were found between AS and SPEM performance. Internal consistency was high for all measures and similar within-session performance changes were observed in both groups for most variables. Most clinical variables did not correlate with AS and SPEM performance. These eye movement measures can therefore function as valid endophenotypes in future studies of potential schizophrenia risk genotypes in the genetically homogenous Icelandic population.
References
Amador XF, Malaspina D, Sackeim HA, Coleman EA, Kaufmann CA, Hasan A, Gorman JM (1995) Visual fixation and smooth pursuit eye movement abnormalities in patients with schizophrenia and their relatives. J Neuropsychiatry Clin Neurosci 7:197–206
Antoniades CA, Altham PM, Mason SL, Barker RA, Carpenter R (2007) Saccadometry: a new tool for evaluating presymptomatic Huntington patients. Neuroreport 18:1133–1136
Braff DL, Freedman R, Schork NJ, Gottesman II (2007) Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull 33:21–32
Broerse A, Crawford TJ, den Boer JA (2001) Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia 39:742–756
Calkins ME, Curtis CE, Iacono WG, Grove WM (2004) Antisaccade performance is impaired in medically and psychiatrically healthy biological relatives of schizophrenia patients. Schizophr Res 71:167–178
Calkins ME, Iacono WG (2000) Eye movement dysfunction in schizophrenia: a heritable characteristic for enhancing phenotype definition. Am J Med Genet 97:72–76
Calkins ME, Iacono WG, Curtis CE (2003) Smooth pursuit and antisaccade performance evidence trait stability in schizophrenia patients and their relatives. Int J Psychophysiol 49:139–146
Cegalis JA, Sweeney JA (1979) Eye movements in schizophrenia: a quantitative analysis. Biol Psychiatry 14:13–26
Cegalis JA, Sweeney JA, Dellis EM (1982) Refixation saccades and attention in schizophrenia. Psychiatry Res 7:189–198
Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS (1999) Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci USA 96:4724–4729
Clementz BA, McDowell JE, Zisook S (1994) Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol 103:277–287
Crawford TJ, Haeger B, Kennard C, Reveley MA, Henderson L (1995) Saccadic abnormalities in psychotic patients. II. The role of neuroleptic treatment. Psychol Med 25:473–483
Crawford TJ, Sharma T, Puri BK, Murray RM, Berridge DM, Lewis SW (1998) Saccadic eye movements in families multiply affected with schizophrenia: the Maudsley Family Study. Am J Psychiatry 155:1703–1710
Cronbach LJ, Warrington WG (1951) Time-limit tests: estimating their reliability and degree of speeding. Psychometrika 16:167–188
Curtis CE, Calkins ME, Iacono WG (2001) Saccadic disinhibition in schizophrenia patients and their first-degree biological relatives. A parametric study of the effects of increasing inhibitory load. Exp Brain Res 137:228–236
Depatie L, O’Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, Lal S (2002) Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology 27:1056–1070
Ettinger U, Kumari V, Crawford TJ, Corr PJ, Das M, Zachariah E, Hughes C, Sumich AL, Rabe-Hesketh S, Sharma T (2004) Smooth pursuit and antisaccade eye movements in siblings discordant for schizophrenia. J Psychiatr Res 38:177–184
Ettinger U, Kumari V, Crawford TJ, Davis RE, Sharma T, Corr PJ (2003) Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology 40:620–628
Ettinger U, Picchioni M, Hall MH, Schulze K, Toulopoulou T, Landau S, Crawford TJ, Murray RM (2006) Antisaccade performance in monozygotic twins discordant for schizophrenia: the Maudsley twin study. Am J Psychiatry 163:543–545
Evans WJ, Schwartz BD (1997) Attentional mechanisms of saccadic eye movements in schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol 10:17–24
Flechtner KM, Steinacher B, Sauer R, Mackert A (2002) Smooth pursuit eye movements of patients with schizophrenia and affective disorder during clinical treatment. Eur Arch Psychiatry Clin Neurosci 252:49–53
Friedman L, Jesberger JA, Meltzer HY (1991) A model of smooth pursuit performance illustrates the relationship between gain, catch-up saccade rate, and catch-up saccade amplitude in normal controls and patients with schizophrenia. Biol Psychiatry 30:537–556
Fukushima J, Morita N, Fukushima K, Chiba T, Tanaka S, Yamashita I (1990) Voluntary control of saccadic eye movements in patients with schizophrenic and affective disorders. J Psychiatr Res 24:9–24
Gooding DC, Grabowski JA, Hendershot CS (2000) Fixation stability in schizophrenia, bipolar, and control subjects. Psychiatry Res 97:119–128
Gooding DC, Iacono WG, Beiser M (1994) Temporal stability of smooth-pursuit eye tracking in first-episode psychosis. Psychophysiology 31:62–67
Harrison PJ, Weinberger DR (2005) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10:40–68; image 45
Helgason A, Yngvadottir B, Hrafnkelsson B, Gulcher J, Stefansson K (2005) An Icelandic example of the impact of population structure on association studies. Nat Genet 37:90–95
Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW (1974) Eye-tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry 31:143–151
Hommer DW, Clem T, Litman R, Pickar D (1991) Maladaptive anticipatory saccades in schizophrenia. Biol Psychiatry 30:779–794
Hong LE, Mitchell BD, Avila MT, Adami H, McMahon RP, Thaker GK (2006) Familial aggregation of eye-tracking endophenotypes in families of schizophrenic patients. Arch Gen Psychiatry 63:259–264
Hong LE, Tagamets M, Avila M, Wonodi I, Holcomb H, Thaker GK (2005) Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biol Psychiatry 57:726–732
Hutton SB, Crawford TJ, Gibbins H, Cuthbert I, Barnes TR, Kennard C, Joyce EM (2001) Short and long term effects of antipsychotic medication on smooth pursuit eye tracking in schizophrenia. Psychopharmacology (Berl) 157:284–291
Hutton SB, Crawford TJ, Kennard C, Barnes TR, Joyce EM (2000) Smooth pursuit eye tracking over a structured background in first-episode schizophrenic patients. Eur Arch Psychiatry Clin Neurosci 250:221–225
Hutton SB, Crawford TJ, Puri BK, Duncan LJ, Chapman M, Kennard C, Barnes TR, Joyce EM (1998) Smooth pursuit and saccadic abnormalities in first-episode schizophrenia. Psychol Med 28:685–692
Hutton SB, Ettinger U (2006) The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology 43:302–313
Kaiser S, Roth A, Rentrop M, Friederich HC, Bender S, Weisbrod M (2008) Intra-individual reaction time variability in schizophrenia, depression and borderline personality disorder. Brain Cogn 66:73–82
Karoumi B, Ventre-Dominey J, Vighetto A, Dalery J, d’Amato T (1998) Saccadic eye movements in schizophrenic patients. Psychiatry Res 77:9–19
Katsanis J, Iacono WG (1991) Clinical, neuropsychological, and brain structural correlates of smooth-pursuit eye tracking performance in chronic schizophrenia. J Abnorm Psychol 100:526–534
Katsanis J, Kortenkamp S, Iacono WG, Grove WM (1997) Antisaccade performance in patients with schizophrenia and affective disorder. J Abnorm Psychol 106:468–472
Katsanis J, Taylor J, Iacono WG, Hammer MA (2000) Heritability of different measures of smooth pursuit eye tracking dysfunction: a study of normal twins. Psychophysiology 37:724–730
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Kelly P, Rennie C, Gordon E, Anderson J, Howson A, Meares R (1990) Smooth pursuit eye tracking dysfunction and negative symptoms in schizophrenia. Psychiatry Res 34:89–97
Kissler J, Clementz BA (1998) Fixation stability among schizophrenia patients. Neuropsychobiology 38:57–62
Klein C, Heinks T, Andresen B, Berg P, Moritz S (2000) Impaired modulation of the saccadic contingent negative variation preceding antisaccades in schizophrenia. Biol Psychiatry 47:978–990
Klein C, Wendling K, Huettner P, Ruder H, Peper M (2006) Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry 60:1088–1097
Krebs MO, Gut-Fayand A, Amado I, Daban C, Bourdel MC, Poirier MF, Berthoz A (2001) Impairment of predictive saccades in schizophrenia. Neuroreport 12:465–469
Leigh R, Zee D (1999) Neurology of eye movments. Oxford University Press, Oxford
Lencer R, Nagel M, Sprenger A, Heide W, Binkofski F (2005) Reduced neuronal activity in the V5 complex underlies smooth-pursuit deficit in schizophrenia: evidence from an fMRI study. Neuroimage 24:1256–1259
Lencer R, Trillenberg-Krecker K, Schwinger E, Arolt V (2003) Schizophrenia spectrum disorders and eye tracking dysfunction in singleton and multiplex schizophrenia families. Schizophr Res 60:33–45
Levy DL, Holzman PS, Matthysse S, Mendell NR (1993) Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophr Bull 19:461–536
Lichtermann D, Karbe E, Maier W (2000) The genetic epidemiology of schizophrenia and of schizophrenia spectrum disorders. Eur Arch Psychiatry Clin Neurosci 250:304–310
Litman RE, Torrey EF, Hommer DW, Radant AR, Pickar D, Weinberger DR (1997) A quantitative analysis of smooth pursuit eye tracking in monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry 54:417–426
Mackert A, Flechtner M (1989) Saccadic reaction times in acute and remitted schizophrenics. Eur Arch Psychiatry Neurol Sci 239:33–38
Malone SM, Iacono WG (2002) Error rate on the antisaccade task: heritability and developmental change in performance among preadolescent and late-adolescent female twin youth. Psychophysiology 39:664–673
Moon SY, Barton JJ, Mikulski S, Polli FE, Cain MS, Vangel M, Hamalainen MS, Manoach DS (2007) Where left becomes right: a magnetoencephalographic study of sensorimotor transformation for antisaccades. Neuroimage 36:1313–1323
Matsue Y, Saito H, Osakabe K, Awata S, Ueno T, Matsuoka H, Chiba H, Fuse Y, Sato M (1994) Smooth pursuit eye movements and voluntary control of saccades in the antisaccade task in schizophrenic patients. Jpn J Psychiatry Neurol 48:13–22
Matsue Y, Sugawara S, Oyama K, Osakabe K, Awata S, Goto Y, Sato M (1993) Smooth pursuit eye movement dysfunction as a biological marker for prediction of disease courses of schizophrenia: a preliminary report. Jpn J Psychiatry Neurol 47:71–74
McDowell JE, Clementz BA (2001) Behavioral and brain imaging studies of saccadic performance in schizophrenia. Biol Psychol 57:5–22
McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA (1999) Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology 36:138–141
Mosimann UP, Felblinger J, Colloby SJ, Muri RM (2004) Verbal instructions and top-down saccade control. Exp Brain Res 159:263–267
Nagel M, Sprenger A, Nitschke M, Zapf S, Heide W, Binkofski F, Lencer R (2007) Different extraretinal neuronal mechanisms of smooth pursuit eye movements in schizophrenia: an fMRI study. Neuroimage 34:300–309
Nkam I, Thibaut F, Denise P, Van Der Elst A, Segard L, Brazo P, Menard J, Thery S, Halbeck I, Delamilleure P, Vasse T, Etard O, Dollfus S, Champion D, Levillain D, Petit M (2001) Saccadic and smooth-pursuit eye movements in deficit and non-deficit schizophrenia. Schizophr Res 48:145–153
Radant AD, Hommer DW (1992) A quantitative analysis of saccades and smooth pursuit during visual pursuit tracking. A comparison of schizophrenics with normals and substance abusing controls. Schizophr Res 6:225–235
Raemaekers M, Jansma JM, Cahn W, Van der Geest JN, van der Linden JA, Kahn RS, Ramsey NF (2002) Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Arch Gen Psychiatry 59:313–320
Reuter B, Kathmann N (2004) Using saccade tasks as a tool to analyze executive dysfunctions in schizophrenia. Acta Psychol (Amst) 115:255–269
Reuter B, Rakusan L, Kathmanna N (2005) Poor antisaccade performance in schizophrenia: an inhibition deficit? Psychiatry Res 135:1–10
Riley B, Kendler KS (2006) Molecular genetic studies of schizophrenia. Eur J Hum Genet 14:669–680
Schlenker R, Cohen R (1995) Smooth-pursuit eye-movement dysfunction and motor control in schizophrenia: a follow-up study. Eur Arch Psychiatry Clin Neurosci 245:125–126
Schwartz BD, O’Brien BA, Evans WJ, McDermott BE, Sautter FJ, Winstead DK Jr (1995) Abnormal saccadic eye movements associated with positive family history schizophrenics. Biol Psychiatry 38:487–491
Sereno AB, Holzman PS (1995) Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry 37:394–401
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33;quiz 34–57
Siever LJ, van Kammen DP, Linnoila M, Alterman I, Hare T, Murphy DL (1986) Smooth pursuit eye movement disorder and its psychobiologic correlates in unmedicated schizophrenics. Biol Psychiatry 21:1167–1174
Smyrnis N, Evdokimidis I, Stefanis NC, Constantinidis TS, Avramopoulos D, Theleritis C, Paximadis C, Efstratiadis C, Kastrinakis G, Stefanis CN (2002) The antisaccade task in a sample of 2,006 young males. II. Effects of task parameters. Exp Brain Res 147:53–63
Spitzer R, Endicott J (1977) The schedule for affective disorders and schizophrenia, lifetime version. NewYork State Psychiatric Institute, New York
Spitzer RL, Endicott J, Robins E (1978) Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry 35:773–782
Sweeney JA, Bauer KS, Keshavan MS, Haas GL, Schooler NR, Kroboth PD (1997) Adverse effects of risperidone on eye movement activity: a comparison of risperidone and haloperidol in antipsychotic-naive schizophrenic patients. Neuropsychopharmacology 16:217–228
Sweeney JA, Clementz BA, Haas GL, Escobar MD, Drake K, Frances AJ (1994) Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity, and the role of attention. J Abnorm Psychol 103:222–230
Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, Carl JR (1998) Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biol Psychiatry 44:698–708
Thaker GK, Avila MT, Hong EL, Medoff DR, Ross DE, Adami HM (2003) A model of smooth pursuit eye movement deficit associated with the schizophrenia phenotype. Psychophysiology 40:277–284
Tien AY, Ross DE, Pearlson G, Strauss ME (1996) Eye movements and psychopathology in schizophrenia and bipolar disorder. J Nerv Ment Dis 184:331–338
Acknowledgments
The study was supported by a grant from the Icelandic Research Fund (RANNIS). Ulrich Ettinger is supported by an ESRC/MRC Fellowship. We would like to thank Dr S.B. Hutton (s.hutton@sussex.ac.uk) for writing the SPEM analysis software.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haraldsson, H.M., Ettinger, U., Magnusdottir, B.B. et al. Eye movement deficits in schizophrenia. Eur Arch Psychiatry Clin Neurosc 258, 373–383 (2008). https://doi.org/10.1007/s00406-008-0806-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-008-0806-y