Abstract
Adults with persistent attention-deficit/hyperactivity disorder (ADHD) may show cognitive deficits as compared to healthy control subjects. The aim of this study was to compare a sample of adult outpatients with ADHD on medication to healthy controls on a comprehensive neuropsychological assessment battery. Thirty adults with ADHD under stable psychopharmacological treatment and 27 healthy controls matched for age, gender, and IQ were assessed with ten tests measuring performance with regard to attention, memory, executive function, and fine motor control. Lower performance in patients as compared to controls was found in tests of verbal and visual memory, speed of visuo-motor search, set shifting, and divided attention. Indicators of response inhibition and simple response speed were less affected. Adults with ADHD show indicators of lowered cognitive performance under medication. These are related more to memory and attention under high mental load than to response inhibition or simple attention or motor performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diagnosis of ADHD in adults received some clinical and scientific interest in the last years. Of about 3–7% of children with ADHD in childhood, ADHD persists into adulthood in about 20–50% [8, 13, 35, 39]. Adults with ADHD show impairments in social and occupational functioning [9] and from the three major symptom dimensions hyperactivity, impulsivity and inattention, the latter seems to be more persistent into adulthood [23].
Neurocognitive functions have been assessed primarily in unmedicated adults with ADHD [22], where patients showed deficits in memory and executive function. Tests of behavioural inhibition have been shown to be only partially impaired in adults [11] and psychopharmacological interventions have been shown to improve cognitive performance measures like response inhibition [1, 40]. Given the impact of cognitive function on deficits in social skills and occupational success, we aimed to assess cognitive performance in a sample of psychopharmacologically treated adult patients with ADHD. Understanding neuropsychological performance of patients in an outpatient ambulance setting may provide insight into persistent cognitive performance deficits and help to further guide additional treatment strategies [17].
Methods
Psychiatric assessment
We assessed 30 adult outpatients with a diagnosis of ADHD (DSM-IV 314.00/314.01) and 27 controls matched for age, gender and education (Table 1). Of 30 patients, ten were diagnosed as predominantly inattentive (DSM-IV 314.00) and 20 patients were diagnosed as combined type (DSM-IV 314.01). None of the patients was predominantly hyperactive-impulsive. Before inclusion to the study, subjects gave written informed consent. ADHD diagnosis was established by a psychiatrist (AKP) and confirmed by a senior psychiatrist (ED). Psychiatric assessments were supported by means of a structured interview on the presence of ADHD symptoms and the Conners Adult ADHD rating scale (CAARS, [7]). Patients were consecutively recruited from the outpatient ambulance for adults with ADHD at the Clinic for Psychiatry and Psychotherapy Essen. All Patients received psychopharmacological treatment: one patient received Venlafaxine, the other 29 Patients were treated with Methylphenidate (Ritalin, Ritalin SR, or Concerta in doses between 5 and 60 mg). Control subjects were recruited by advertisements in stores and community centers in Essen.
Self ratings comprised the short version of the Wender Utah Rating scale [42], the self-rating scale for ADHD symptoms [29], and scales for the assessment of anxiety: State-Trait Anxiety Inventory, STAI [19, 36], depression: Beck Depression Inventory, BDI [4], and impulsivity: Barratt Impulsivity Scale, BIS–11 [24]. An IQ-estimate of crystallized general intelligence was assessed by means of the MWT-B test [20]. All scales where given to patients as well as to healthy control subjects. The study was approved by the ethics committee of the faculty of medicine of the University of Duisburg-Essen.
Neuropsychological assessment
The neuropsychological assessment comprised ten tests measuring cognitive function with regard to attention, executive function, and memory performance. The neuropsychological assessment was done in one session which lasted about 1.5 h. Subjects where given the opportunity to take breaks as they needed.
Stroop test
The stroop tests measures the ability to inhibit reactions [38]. Subjects have to read lists of colour words, name colours, and name colours of incongruent colour words. Measures for reading time (nomination) and slowed reading time in the incongruence condition (selection) and errors were scored [3].
Controlled Oral Word Association (COWA)
This tests measures verbal fluency [5]. Subjects are asked to say as many word as they can think of, beginning with the letters F, A, and S, each within a minute of time. Proper nouns, names of people, and variations of a word with different suffixes are not allowed. The test result gives the number of correct words.
Tower of London
This computer aided test measures the ability to plan the sorting of balls in order to achieve a given configuration [34]. Here we used a computer aided test form developed by Schall et al. [32]. The test gives the time to the first movement, the time needed for the movements, and the total time used for problem solving.
Visual reproduction and logical memory (WMS-R)
These subtests of the Wechsler Memory Scale Revised measure visual and verbal reproduction [43]. Four cards displaying geometric figures and two short stories have to be reproduced immediately and after 30 min. Scores represent the number of correctly reproduced elements.
Trails A and B
The trail making test measures visual scanning and cognitive flexibility. Part A of the trail making test consists of a random display of numbers, which have to be connected in sequence. Part B requires alterations between numbers and letters. Scores represent time for task completion [37].
Motor performance (MLS)
The motor performance test combines a series of tasks measuring fine motor performance [14, 33]. Here we used a combination of four subtasks where subjects had to steadily hold a pen into a hole, follow a line with a pen, quickly tap a series of points in a row, and quickly tap the pen on a plate. Each task was performed with the right and the left hand. Data were combined according to measures of tremor, precision, and speed of hand and finger movements [12].
Divided attention task (TAP)
This subtest of the computer aided battery for the assessment of attention [46] measures the ability to simultaneously perform an auditory and a visual target detection task [18]. Subjects have to press a button each time an alternating tone is repeated twice or four dots build a square configuration on the computer screen. This tests measures reaction time and number of correct responses to visual and auditory targets.
Covered orienting of attention (TAP)
This subtest of the computer aided test battery for the assessment of attention [46] consists of a simple reaction time task with a preceding cue in the center of the screen pointing with high probability to the side where the target stimulus will appear (valid cues: 80%) or in rare cases to the opposed side (invalid cues: 20%). The ability to effectively shift attention to the expected target point is evaluated by the reaction time of valid cued trials [25].
Sensomotor function (Neurobat)
The computer aided Neurobat subtest “sensomotor function” measures the inhibition of overlearned visuomotor reactions. The letters R(ight) and L(eft) are displayed left and right of a fixation point. Subjects are required to respond to the direction indicated by the letter while ignoring the information provided by the position of the letter relative to the fixation point [44].
Sustained Attention (Macworth-Clock, Neurobat)
The Neurobat subtest “sustained attention” is a computer aided implementation of the Mackworth-Clock measuring sustained attention and the ability to search for and correctly respond to rare events in the visual domain [21]. Here we used a computerized version of this test where a white dot continuously jumps from one to the next place in a circle. Subjects had to respond to rare events of the dot moving two steps at a time. This test provides mean reaction times taken from three consecutive periods of time within test completion and the total number of errors of commission and errors of omission [44].
Data analysis
Patients and control subjects were compared with regard to basic subject characteristics using univariate analysis of variance and chi2-tests. Rating and self-rating scores on clinical variables were compared only with regard to total scores using univariate analysis of variance. Dependent variables from neuropsychological tests were analyzed and compared to the matched control group data, using raw test scores where applicable and univariate analysis of variance, and results at P < 0.01 are considered as relevant. In order to estimate the relevance of the results, partial Eta2 coefficients are reported. Eta2 coefficients give the amount of variance explained by the group factor [6]. Effect sizes at .1 are considered as small, at .3 as medium, and at .5 as large. The influence of anxiety and depression on cognitive performance data were analyzed with analysis of covariance using BDI and STAI sum-scores. Results are reported where they had an impact on cognitive test results.
Results
Adult patients with persistent ADHD and control subjects did not differ with regard to age, gender, years in education, handedness, and IQ. In contrast to these more general characteristics, indicators of clinical symptoms differed highly between groups. Means, and standard deviations are given in Table 1. Largest differences between patients and controls were found with regard to symptoms of attention on the ADHD DSM-IV symptom ratings, the ADHD self rating questionnaire and the Conners Adult ADHD rating scale. Patients with ADHD scored significantly higher in these clinical symptom dimensions. Symptoms of hyperactive behavior and impulsivity were significantly higher than in the controls but less prominent when compared to the symptoms of attention. Although patients and controls differed significantly with regard to measures of anxiety (STAI), depression (BDI), and impulsivity (BIS) and on the inconsistency index of the Connors Adult ADHD rating scale, these differences between patients and controls were much lower when compared to the differences in ADHD-related symptom scores. Within the State-Trait Anxiety Inventory, trait anxiety exceeded state anxiety in patients with ADHD but not in controls (multivariate analysis of variance [MANOVA] interaction group × subtest F[1, 54] = 8.3, P = .006).
The analysis of anxiety and depression scores as covariates in the neuropsychological test results revealed relevant associations in three tests: In the Tower of London task, time to first movement and the total time used were associated with anxiety scores. The introduction of the covariate led to an increase in partial Eta2 from .10 to .18 with the first time to movement and from .12 to .24 with the total time used. In the fine motor performance, anxiety and depression scores lowered the non-significant differences with regard to tremor and precision performance. Depression scores were associated with correct right/right reactions in the covered orienting of attention task. Here, the covariate did not alter the significance of the performance difference between patients and controls.
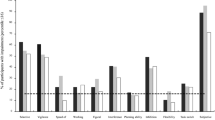
Detailed results of the neuropsychological assessment are summarized in Table 2. Differences between patients and controls emerged in the verbal and in the visual reproduction subtest of the Wechsler Memory Scale Revised, where patients showed deficits with large effect sizes with partial Eta2 at about .5 (Fig. 1a, b).
Cognitive performance in adults with persistent ADHD and healthy control subjects: (a) Wechsler Memory Scale Revised subtest logical memory and (b) subtest visual reproduction. Immediate and 30 min delayed reproduction test scores. (c) Trail making test part A (numbers) and part B (numbers and letters) performance
Lower performance in patients as compared to controls was also found in the trail making test in part A and B (Fig. 1c). Visual search in the trail making test part A was slower in patients as compared to controls. In part B, parallel processing of numbers and letters led to a heightened performance loss in patients as compared to control subjects as indicated by the significant two-way interaction between groups (ADHD/controls) × subtests (part A/B): F[1, 55] = 13.7, P < .001. However, due to higher variances in the patient group in the trail making test part B effect sizes were in the medium range for both subtests.
In the divided attention task patients showed slower reaction times. The amount of reaction time slowing tended to be slightly larger in the visual as compared to the auditory domain. However, the multivariate analysis of variance did not show a significant group (ADHD/control) × domain (auditory/visual) effect (F[1, 55] = 1.8, P = .187). The number of errors to auditory stimuli was higher in patients than in controls, but there was no difference between patients and controls in errors to visual stimuli.
In the covered orienting of attention task we found overall slowed reaction time in patients with persistent ADHD. Invalid cues led to higher reaction times in patients as compared to controls. In a multivariate analysis of variance combining group, laterality, and validity of cues we found a significant two-way interaction between group and cue validity (F[1,55]=8.1, P = .006). No significant effects were found with regard to other interaction effects involving the group factor. Patients made more errors on valid cued targets on the right then on valid left or invalid left or right cued targets.
In the sensomotor function task, the effects of left/right compatible and incompatible target stimuli were compared. Patients showed slower reaction times in all but the left compatible condition. The multivariate analysis of variance combining group, target location, and compatibility of arrow and location indicated no relevant interaction effects involving the group factor. However, patients tended to show more errors (Table 2).
In the Tower of London task, patients took more time to think until the start of the first movement and more for the total time. There was no interaction in the time to plan and the action itself.
In the Stroop test we found no indication of slowed processing time in patients to simple or incompatible stimuli. Patients made more errors when they had to name the colours of incompatible printed colour words. In the controlled oral word association test, controls produced some more words as compared to patients with ADHD (Table 2).
Tests without significant group effects comprised the fine motor performance tasks and the Macworth Clock. Reaction times to simple rare target events did not differ between patients and controls and we found no differences in errors or commission and errors of omission. Furthermore, we found no indication of increased reaction times within the course of the three consecutive phases of this test.
Discussion
In our study we assessed a sample of adult outpatients with persistent symptoms of ADHD and a group of healthy control subjects matched for age, gender, education, and IQ. All patients were under psychopharmacological treatment. In contrast to typical samples of children and adolescents with ADHD, in our sample, clinical symptoms of adult patients were largely related to problems of inattention and less to symptoms of hyperactivity or impulsiveness.
Anxiety scores in ADHD patients were more than one standard deviation higher than those of controls in our study. Trait anxiety scores were in the range of patients with specific phobias but more than one standard deviation lower than in patients with generalized anxiety [19]. Although BDI scores in ADHD patients were considerably higher when compared to controls, mean scores are lower than those considered to indicate clinical relevant depression symptoms [15]. They are, however, in line with results obtained in patients who are subject to heightened distress like chronic pain [16]. The impulsivity scores in the Barratt Impulsivity Scale in our controls was lower than those of undergraduates; patients’ scores where higher than those of psychiatric patients in the Patton et al. (1995) sample. Although the Barratt impulsivity scale is not designed to assess impulsivity as a specific symptom dimension in ADHD, impulsivity may be less prominent when compared to attention symptoms in adults. This result supports the notion of heightened impulsivity as one of the clinical characteristics in our sample [24].
In the analysis of our results, covariance analyses were performed in order to control for effects of anxiety and depression on cognitive performance. While the association of depression with the number of correct responses in one of the four conditions did not affect results, in the Tower of London task, the introduction of anxiety scores into the analysis raised the effect-size of the performance difference between patients and controls with regard to the time used until the first movement was initiated and with regard to the total time used to solve the task. An attenuation of differences was seen only in two fine motor performance tasks, where non-significant differences between patients and controls were reduced. Therefore, there is no indication that lowered performance in patients with persistent ADHD may be due to detrimental effects that anxiety or depression symptoms may have on cognitive performance.
While the retrospective assessment of ADHD symptoms in childhood using the German version of the Wender Utah Rating Scale short form indicated a high amount of ADHD-related symptoms in our patient sample [27], this clinical sample may not be representative for all adults with persistent ADHD. It may, however, be typical to patients who seek help in a professional outpatient setting [9, 26, 28, 31]. While psychopharmacological treatment in adults may improve cognitive performance with regard to response inhibition [40], the neuropsychological assessment of our study gives an impression on persistent cognitive deficits under psychopharmacological treatment.
The neuropsychological assessment comprised measures of memory performance, including tests of immediate and delayed verbal and visual reproduction, measures of executive function, including tests of verbal fluency, response inhibition, planning, and fine motor control, and measures of attention, including divided attention, covered orienting, and sustained attention. Surprisingly, a large difference in performance of patients and controls was found in the visual reproduction task of the Wechsler Memory Scale Revised, whereas verbal memory was affected to a lesser extent. A difference in performance between patients and controls was found in the trail making test. Relative to controls, patients took more time in the visual scanning of numbers and showed a decrease in performance in the condition of simultaneous handling of letters and numbers. Taken together with slowed reaction times in the divided attention task and slowed performance in the Tower of London task, our results indicate that patients demonstrate lowered performance in executive functions which may not be present with simple reaction time tasks like the Macworth-Clock, but may emerge under conditions of heightened mental load when attention has to be focussed on simultaneous tasks.
This view is supported by the results of the other tests of the assessment battery. Fine motor function and simple reaction times in the vigilance task where not different from controls in our sample. In the vigilance task we also found no indication for an increase in reaction time during the course of the three consecutive test phases. The lack of any difference between patients and controls in the fine motor performance tests is in accordance with low symptoms of hyperactivity.
Another surprising result in the assessment of our patient sample was the low amount of inhibition problems. The Stroop task performance, which may be considered as one of the primary neuropsychological test indicators in children with ADHD [30], was only partly impaired in our sample of adults. In the Stroop task, patients made more errors than controls but we found no prominent indication of altered processing speed in our patients. When compared to the results found in the memory and the trail making tasks, in the inhibition task, differences in Stroop performance between patients and controls were low. There was, however, an interaction between the validity of cues and groups with regard to reaction times in the covered orienting of attention task. With invalid cues, subjects have to reorient attention to the opposite side. In our study, patients took some more time with the process of shifting attention.
Inhibition of overlearned reactions as in the Stroop task is more related to cingulate function [45], whereas the reorienting of attention in response to invalid cues is more associated with right parietal and right frontal cortical areas [41]. Therefore, our results support the hypothesis of a stabilized inhibition system and unimpaired motor function in adults with ADHD as compared to neuropsychological test results in children [2, 10]. While behavior inhibition may have been stabilized by means of pharmacological medication [1, 40], our results point towards a persistent lowered right fronto-parietal functioning under stable medication. This may lead to limitations in attention-dependent cognitive abilities, which here emerge as deficit in the ability to process two discrimination tasks simultaneously and to effectively encode and reactivate memories. Translated to every day functioning, these decrements in performance may become evident in complex social situations, where concurrent interests have to be reacted to in private or occupational fields or in situations when quick decisions are required in the monitoring of multiple information.
In summary, in medicated adult patients with persistent ADHD, we found deficits in visual memory, executive function, and in attention functions with high cognitive load. In patients, we found no or only minor performance decrements in simple discrimination tasks, in reaction time tasks, and in assessments of fine motor function. Psychopharmacologically treated adults in an outpatient ambulance may differ from children and adolescents with regard to less symptoms of hyperactivity und impulsiveness. In our study, symptoms related to anxiety and depression were elevated, but on average remained below clinical relevant thresholds and did not explain differences between patients and controls. Visual memory, executive function, and divided attention deficits may have to be taken into account when planning interventions or coping strategies in order to increase cognitive function under heightened challenges in the patients daily living.
References
Aron AR, Dowson JH, Sahakian BJ, Robbins TW (2003) Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 54:1465–1468
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121:65–94
Bäumler G (1985) Farbe-Wort-Interferenztest (FWIT) nach J.R. Stroop. Hogrefe Verlag, Göttingen
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Benton AL, Hamsher KdS (1989) Multilingual aphasia examination. AJA Associates, Iowa City, Iowa
Cohen J (1977) Statistical power analysis for the behavioral sciences. Academic Press, San Diego
Conners CK, Erhardt D, Sparrow E (1999) Conners’ adult ADHD ratings scales (CAARS). Multi-Health Systems, North Tonawanda, NY
Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH (2002) Conduct problems, gender and adult psychiatric outcome of children with attention-deficit hyperactivity disorder. Brit J Psychiatry 181:416–421
Davids E, Krause DA, Specka M, Gastpar M (2004) [Analysis of a special consultation for attention deficit/hyperactivity disorder in adults]. Gesundheitswesen 66:416–422
Davids E, Zhang K, Tarazi FI, Baldessarini RJ (2003) Animal models of attention-deficit hyperactivity disorder. Brain Res Brain Res Rev 42:1–21
Epstein JN, Johnson DE, Varia IM, Conners CK (2001) Neuropsychological assessment of response inhibition in adults with ADHD. J Clin Exp Neuropsychol 23:362–371
Fleishmann EA (1972) Structure and measurement of psychomotor abilities. In: Singer RN (ed) The psychomotor domain. Lea & Febiger, Philadelphia, pp 78–106
Ford T, Goodman R, Meltzer H (2003) The British Child and Adolescent Mental Health Survey 1999: the prevalence of DSM-IV disorders. J Am Acad Child Adolesc Psychiatry 42:1203–1211
Hamster W (1980) Klinische Normen zur Motorischen Leistungsseries. Dr. G. Schuhfried GmbH, Mölding, Austria
Hautzinger M (1991) Das Beck-Depressionsinventar (BDI) in der Klinik. Der Nervenarzt 62:689–696
Hautzinger M, Bailer M, Worall H, Keller F (1995) Beck-Depressions-Inventar (BDI) Bearbeitung der deutschen Ausgabe. Verlag Hans Huber, Göttingen
Hesslinger B, Tebartz van Elst L, Nyberg E, Dykierek P, Richter H, Berner M, Ebert D (2002) Psychotherapy of attention deficit hyperactivity disorder in adults–a pilot study using a structured skills training program. Eur Arch Psychiatry Clin Neurosci 252:177–184
Kahnemann D (1973) Attention and effort. Prentice-Hall, Englewood Cliffs, New Jersey
Laux L, Glanzmann P, Schaffner P, Spielberger CD (1981) Das State-Trait-Angstinventar. Beltz, Weinheim
Lehrl S (1995) Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. PERMED-spitta Medizinische Verlagsgesellschaft mbH, Balingen
Mackworth NH (1948) The breakdown of vigilance during prolonged visual search. Q J Exp Psychol 1:6–21
McLean A, Dowson J, Toone B, Young S, Bazanis E, Robbins TW, Sahakian BJ (2004) Characteristic neurocognitive profile associated with adult attention-deficit/hyperactivity disorder. Psychol Med 34:681–692
Mick E, Faraone SV, Biederman J (2004) Age-dependent expression of attention-deficit/hyperactivity disorder symptoms. Psychiatr Clin North Am 27:215–224
Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774
Posner MI, Petersen SE (1990) The attention system of the human brain. Ann Rev Neurosci 13:25–42
Retz W, Retz-Junginger P, Hengesch G, Schneider M, Thome J, Pajonk FG, Salahi-Disfan A, Rees O, Wender PH, Rösler M (2004) Psychometric and psychopathological characterization of young male prison inmates with and without attention deficit/hyperactivity disorder. Eur Arch Psychiatry Clin Neurosci 254:201–208
Retz-Junginger P, Retz W, Blocher D, Stieglitz RD, Georg T, Supprian T, Wender PH, Rosler M (2003) [Reliability and validity of the Wender-Utah-Rating-Scale short form. Retrospective assessment of symptoms for attention deficit/huperactivity disorder]. Nervenarzt 74:987–993
Rösler M, Retz W, Retz-Junginger P, Hengesch G, Schneider M, Supprian T, Schwitzgebel P, Pinhard K, Dovi-Akue N, Wender P, Thome J (2004) Prevalence of attention deficit-/hyperactivity disorder (ADHD) and comorbid disorders in young male prison inmates*. Eur Arch Psychiatry Clin Neurosci 254:365–371
Rösler M, Retz W, Retz-Junginger P, Thome J, Supprian T, Nissen T, Stieglitz RD, Blocher D, Hengesch G, Trott GE (2004) [Tools for the diagnosis of attention-deficit/hyperactivity disorder in adults. Self-rating behaviour questionnaire and diagnostic checklist. Nervenarzt 75:888–895
Rubia K, Taylor E, Smith AB, Oksanen H, Overmeyer S, Newman S (2001) Neuropsychological analyses of impulsiveness in childhood hyperactivity. Brit J Psychiatry 179: 138–143
Sayal K, Taylor E, Beecham J, Byrne P (2002) Pathways to care in children at risk of attention-deficit hyperactivity disorder. Brit J Psychiatry 181:43–48
Schall U, Johnston P, Lagopoulos J, Juptner M, Jentzen W, Thienel R, Dittmann-Balcar A, Bender S, Ward PB (2003) Functional brain maps of Tower of London performance: a positron emission tomography and functional magnetic resonance imaging study. Neuroimage 20:1154–1161
Schoppe KJ (1974) Das MLS-Gerät: ein neuer Testapparat zur Messung feinmotorischer Leistungen. Diagnostica 20:43–47
Shallice T (1982) Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298:199–209
Spencer T, Biederman J, Wilens T, Faraone SV (1994) Is attention-deficit hyperactivity disorder in adults a valid disorder? Harv Rev Psychiatry 1:326–335
Spielberger CD, Gosruch RL, Lushene RE (1970) STAI, Manual for the State-Trait-Anxiety-Inventory. Consulting Psychologist Press, Palo Alto
Spreen O, Strauss E (1991) A compendium of neuropsychological tests. Oxford University Press, New York
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662
Thapar A, Harrington R, McGuffin P (2001) Examining the comorbidity of ADHD-related behaviours and conduct problems using a twin study design. Brit J Psychiatry 179:224–229
Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ (2004) Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 55:1031–1040
Vossel S, Thiel CM, Fink GR (2006) Cue validity modulates the neural correlates of covert endogenous orienting of attention in parietal and frontal cortex. Neuroimage 32:1257–1264
Ward MF, Wender PH, Reimherr FW (1993) The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 150:885–890
Wechsler D (1987) Wechsler Memory Scale - Revised. The Psychological Corporation / Harcourt Brace Jovanovich, San Antonio, TX
Wiebel B, Happe A, Piekara FH (1995) Das neuropsychologische Testprogramm TESTBAT (the neuropsychological test battery TESTBAT). PSYMED, Dülmen
Woodward TS, Ruff CC, Ngan ET (2006) Short- and long-term changes in anterior cingulate activation during resolution of task-set competition. Brain Res 1068:161–169
Zimmermann P, Fimm B (2002) A test battery for attentional performance. In: Leclercq M, Zimmermann P (eds) Applied Neuropsychology of Attention. Theory, Diagnosis and Rehabilitation. Psychology Press, London, pp 101–151
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, B.W., Gimbel, K., Keller-Pließnig, A. et al. Neuropsychological assessment of adult patients with attention-deficit/hyperactivity disorder. Eur Arch Psychiatry Clin Neurosci 257, 112–119 (2007). https://doi.org/10.1007/s00406-006-0688-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-006-0688-9