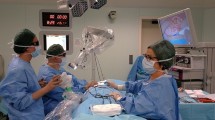
Abstract
Purpose
To define the interest, advantages, and disadvantages of the use of a 3D-exoscope in paediatric ENT surgery.
Methods
Four surgeons with experience in paediatric surgery completed a questionnaire following each surgery performed under 3D-exoscope to evaluate the contribution of the tool compared to the usual practice (microscope or magnifying loupes). Surgeries were separated into three groups: otology, transoral and cleft palate surgery, and open head and neck surgery.
Results
Between June 2021 and June 2022, 151 paediatric surgeries were included in this study. Among them, 93 (62%) otologic surgeries, 35 (23%) transoral surgeries, and 23 (15%) head and neck surgeries were performed. The median age at surgery was 68 months (interquartile range 19–135 months). For otologic surgeries, the mean scores (/100) for the contribution of the exoscope compared to the microscope were 68.4(± 23.2). For transoral and cleft palate surgery, the mean score (/100) for the contribution of the use of the exoscope compared to the magnifying loupes was 92.9 (± 8.6), whereas for open head and neck surgeries, the mean score (/100) was 89.5 (± 7.2).
Conclusion
3D-exoscopy appears to be a relevant tool for paediatric head and neck surgery, applicable in otologic, transoral, and cervical fields. It presents educational and ergonomic advantages and improves surgical team communication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in exoscopy (especially in 3D) is growing in several surgical specialties, especially in neurosurgery and ear, nose and throat (ENT) surgery [1,2,3]. Initially designed to offer surgeons an alternative to traditional operating microscopes and endoscopes, 3D-exoscopy has been used in several surgical fields in ENT with satisfactory and promising results [4,5,6,7,8,9]. Several publications have reported the advantages and inconveniences of this new technology in adult ENT surgery; however, only one paediatric series has been published to date, focusing only on cochlear implantation [10].
3D-exoscopy presents advantages similar to that of microscopy and distinct from endoscopy: depth of the surgical field, identification of anatomical structures, longer focal length (creating a wider working space), and handling of the camera that allows the surgeon to explore the surgical field without the anatomical constraints of microscopy [11]. Moreover, in comparison with microscopy, the transmission on a 4K 3D screen, and the reduced size of the exoscope, offers the surgeon a more ergonomic position and facilitates the transmission of knowledge to students [12].
The main goal of this study was to evaluate the interest in 3D-exoscopy in paediatric ENT surgery.
Materials and methods
Study population
Children were enrolled in this monocentric prospective study in a tertiary department of paediatric otolaryngology conducted between June 2021 and June 2022. All consecutive surgeries were perfomed using the Vitom 3D-exoscope (Karl Storz Company, Tuttlingen, Germany). Surgeries were divided into three categories: otologic, transoral, and open head and neck surgery. Considering the experience of our centre concerning the use of the 3D-exoscope in otology, otologic surgeries were divided into two groups: the first group with chronic otitis and inflammatory tissues (Group 1) and another group with hearing implantation (and therefore a wider surgical field) (Group 2). Independently of the age of the child, surgeries in which the operative technique required magnification of the surgical field (microscope or magnification surgical loupes) were eligible in this study. Four senior surgeons with experience in surgery under microscope or magnification surgical loupes participated in this study.
The investigation adhered to the principles of the Declaration of Helsinki and was approved by our Institutional Human Experimentation Committee (Institutional Review Board 00006477).
Outcome measures
Immediately after each surgery performed using the 3D-exoscope, the surgeon completed a survey to evaluate different aspects of the surgery compared to the usual microscope or magnification surgical loupes. Responses were given using a five-point Likert Scale assessing the set-up and handling of the exoscope, the identification of anatomical structures and pathological tissues, the lighting, the magnification, and the depth of the surgical field. The surgeon's comfort was also evaluated by inquiring about headaches or eye fatigue related to 3D vision and the use of 3D glasses. Finally, the surgeon estimated on a scale of 0 to 100 the contribution of the exoscope compared to usual practice. The complete version of the questionnaire is provided in Supplementary informations 1. The reference technique for magnification of the surgical field was: (1) for otology, a microscope; (2) for transoral and cleft palate surgery, magnifying loupes; and (3) for open head and neck surgery, a microscope or magnifying loupes depending on the surgery. Surgeries that did not require any surgical field magnification techniques were excluded from the study.
Data on operating times were also collected. As cochlear implant surgery is highly standardized, we retrospectively collected operating room occupancy time data from previous consecutive cochlear implant surgeries performed by the same four surgeons with a standard surgical microscope between November 2020 and June 2021, to compare with the operating time of cochlear implant surgeries performed with the 3D-exoscope.
Statistical analysis
Descriptive statistics and statistical analyses were performed using R Statistics software. Data are shown as n (%) or as mean (± SD). Concerning the bivariate analysis, a Student’s t test was used according to the validity conditions. A p value of less than 0.05 was considered to be statistically significant.
Results
General population
Between June 2021 and June 2022, 151 paediatric surgeries were included in this study. Among them, 93 (62%) otologic surgeries, 35 (23%) transoral surgeries, and 23 (15%) head and neck surgeries were performed. The details of the surgical indications performed are presented in Table 1. The median age at surgery was 68 months (interquartile range: 19–135 months) (Fig. 1), with 72 (48%) females and 79 (52%) males.
Otologic surgery
Among the 93 otology surgeries, Group 1 (narrow surgical field or inflammatory tissues with all types of tympanoplasties and middle ear tumours) included 46 (49%) surgeries. Group 2 included 47 (51%) surgeries, mainly composed of cochlear implant surgery (44, 94%) and only three (6%) middle ear implants (Fig. 2A, B). The median age (interquartile range) of the operated children was 152 (107–184) and 38 (23–65) months for Groups 1 and 2, respectively (p value < 0.0001).
Intraoperative pictures with 3D-exoscope. A Cochlear implant surgery before opening the round window. I incus, rw round window. B Middle ear implant surgery. i incus, mea middle ear implant. C Velopharyngoplasty. ppa palatopharyngeal arch, t tonsils, u uvula. D Parotidectomy. Fbcc first branchial cleft cyst, fn facial nerve
In Group 1, surgeons reported that the use of the exoscope was not as efficient as the microscope in approximately 30–35% of cases for anatomical structure identification, lighting and depth of the surgical field, and handling of the camera. In addition, for 22 surgeries in Group 1 (47%), the magnification of the surgical field was worse with the exoscope compared to the microscope.
In Group 2, the use of the exoscope was better in 53 and 68% for the lighting and depth of the surgical field and for the handling and set-up of the camera. Regarding the identification of anatomical structures and magnification of the surgical field, the use of the exoscope was either similar (64–68%) or better (30%).
The mean scores (/100) for the contribution of the exoscope compared to the microscope were 62.1 (± 26.7) for Group 1 and 74.6 (± 17.2) for Group 2 (p value = 0.009). No intraoperative equipment-related complication was reported in both groups.
Considering the standardization of cochlear implant surgeries, we compared operating room occupancy times for surgeries performed under the microscope (n = 18) and under the 3D-exoscope (n = 41, excluding simultaneous bilateral cochlear implantations, between June 2021 and June 2022). No significant difference was observed between the two groups: 210.8 (± 52.2) minutes for the microscopy group and 211.7 (± 50.3) minutes for the exoscope group (p value = 0.95).
Only one surgery (a cochlear implantation performed in June 2021, in the first month of inclusion of this study) required switching from the exoscope to the microscope, due to eye strain. All other otologic surgeries (92/93, 99%) were performed entirely under 3D exoscopic visualisation.
Regarding the comments specific to otology reported by surgeons, the use of the exoscope has the advantage of easily switching from exoscopy to endoscopy through the use of the same video column, as opposed to microscopy and endoscopy, which requires more manoeuvres and loss of time. However, as suggested by the significant difference between Group 1 and Group 2 (Table 2), the performance of the exoscope in an inflammatory environment or in the case of a narrow operating field is slightly worse, in particular because of the high magnification pixelization (Videos 1 and 2).
Cleft and transoral surgery
Thirty-five surgeries, including 29 cleft lip and palate surgeries (83%), were performed with 3D-exoscope (Fig. 2C). The median age (interquartile range) of the operated children was 54 (5–77) months.
The use of the exoscope was found to be better in 94 to 97% of the surgeries compared to magnifying loupes for all criteria of the Likert scale. The mean score (/100) for the contribution of the use of the exoscope compared to the magnifying loupes was 92.9 (± 8.6) (Table 3). No intraoperative equipment-related complication was identified.
Regarding the comments specific to transoral surgery reported by surgeons, the use of the exoscope allows the visualisation of areas that are usually difficult to access (anterior palate, retroalveolar region), without having an uncomfortable and an unergonomic position, which favours a better velo-palatal reconstruction. In addition, the operating field being narrow, the diffusion of the image on screen facilitates the work of the operating assistant, who is able to share the same surgical view as the operator.
Open head and neck surgery
Twenty-three surgeries, with a wide variety of indications, were performed with the 3D-exoscope (Fig. 2D). The median age (interquartile range) of the operated children was 33 (9–98) months.
Regarding the magnification of the surgical field and the handling and installation of the camera, the use of the exoscope was better in 74 to 83% of the surgeries. It was also better in 91 to 96% regarding the identification of anatomical structures (in particular the nerves or parathyroid glands, such as in thyroidectomy or parotidectomy), depth and lightning of the operating field. The mean score (/100) for the contribution of the use of the 3D-exoscope compared to the magnifying loupes and microscope was 89.5 (± 7.2) (Table 3). No intraoperative equipment-related complication was identified.
Comments on the use of the 3D-exoscope in head and neck surgeries report that it allows a more ergonomic posture and provides better visibility for the operating assistant. Moreover, the identification of anatomical structures was facilitated (especially nerves and vessels) by an augmented reality perception due to increased contrast compared to real life (Video 3).
Evolution of the score according to the experience with the 3D-exoscope
As experience with the use of the exoscope increased for the four surgeons in the study, a significant improvement was noted in the scores attributed compared to standard practice (p < 0.001 between the first 2 months of experience and the last 2 months of experience in the study). A similar analysis was performed for cochlear implant surgeries, with identical results (p = 0.001) (Figs. 3, 4).
General comments on the use of the 3D-exoscope
Advantages with the use of the 3D-exoscope
One of the main advantages reported by surgeons of the 3D-exoscope is the educational interest. All participants involved in the operating room (surgeons, anaesthesiologists, nurses, residents, and students) benefit from the same visualisation of the operating field as that of the main surgeon. Moreover, the operating aid is facilitated. The alternation between direct vision and that on the exoscope retransmission screen is rendered easy because of the small steric size of the camera; furthermore, the glasses do not impede direct vision of the surgical field, as opposed to virtual reality glasses. The manoeuvrability of the camera during the surgery is readily managed by the surgeon; it allows for greater freedom of movement and facilitates the installation of the patient at the beginning of the surgery. Finally, the use of the exoscope allows a more ergonomic posture.
Disadvantages with the use of the 3D-exoscope
High magnification of the surgical field can sometimes induce pixelization that alters the identification of the different anatomical structures (especially in otologic surgeries). The use of 3D glasses for long surgeries can induce headaches and eye strain (1/151, 0.7% in our series). For bilateral simultaneous surgeries (e.g., bilateral cochlear implantation), the pre-operative set-up can sometimes be tricky with the risk of desterilization of the operating field.
Discussion
This study reports the largest prospective series of surgeries performed with the 3D-exoscope in paediatric ENT (n = 151). It is also the first paediatric surgery series other than cochlear implantation assessing the benefits of the exoscope. This study performed an evaluation of the use of the exoscope compared to standard magnifying techniques. The results indicate promising use of the exoscope in several areas of paediatric ENT surgery, independently of the age of the child, regarding otology surgery, open head and neck and transoral/cleft surgery; for the latter, this is the first study to describe it in children.
Use of the 4K 3D-exoscope adapted to paediatric ENT surgery as for adults
General use of the exoscope
Developed as an alternative to microscopy, the main advantage of 4K 3D-exoscopy is that it is an excellent educational tool that allows the entire operating room to follow the procedure on the same screen as the surgeon. Moreover, it allows better communication between the operating assistant and the surgeon concerning the positioning and the exchange of surgical instruments [11, 12]. The retransmission of the image on a screen and the manoeuvrability of the camera allow the surgeon to benefit from a more ergonomic and physiological position. Certain angles of view with the microscope sometimes imply very uncomfortable positions, potentially responsible for muscle strains in the case of long operating times [11, 12].
As suggested by our results (concerning cochlear implantation surgery), the use of 3D-exoscope compared to the usual magnification techniques does not seem to increase the duration of the operating time (for the pre-operative set-up as well as for the duration of the surgery) [9, 13, 14]. Concerning surgery with a microscope, the costs of consumables are similar to those of the 3D-exoscope; however, the initial cost of the exoscope is cheaper than that of a surgical microscope [7].
For some operators, excessive visual fatigue due to the use of 3D glasses throughout the procedure can lead to headaches; this was rare (1/151) in our series. No study has yet compared the occurrence of headaches and visual fatigue following prolonged use of the exoscope or microscope [6, 8, 15].
Otology and exoscope
For otologic surgeries, surgeons suggest that the exoscope seems to be at least as interesting a tool as the microscope. However, the exoscope seems to be significantly less efficient when the surgical field is narrow (particularly external auditory canal, oval window) or if the tissues are inflammatory (chronic otitis media), probably due to pixilation at high magnification. Moreover, some surgeons report that tympanic reconstruction (especially ossiculoplasty) seems to be more delicate with the exoscope. These results are in agreement with the data in the literature concerning adult otologic surgery [9, 16, 17].
Head and neck surgery and transoral surgery and exoscope
The feedback from the surgeons in our series for transoral surgery reports an excellent benefit with the 3D-exoscope in this indication, compared to the use of magnifying loupes. As opposed to magnifying loupes, the position is not conditioned by the depth of the surgical field that depends on the lenses; the surgeon can choose a comfortable position and adjust the focus to have perfect magnification, particularly in cases of precise surface surgery (cleft lip).
Crosetti et al. reported in 2020 a series of patients with oropharyngeal malignancies treated transorally with the 3D-exoscope. This study defined the 3D-exoscope as a versatile and compact optical instrument that provides an excellent 3D image, easy to use in surgical routine with a satisfactory depth of operative field [18].
Similarly to transoral surgery, the 3D-exoscope seems to facilitate the surgery in an important way in head and neck surgeries. Several articles have described the use of the 3D-exoscope. Its use in rhinoplasty surgery has shown a clear improvement in the identification of different anatomical structures allowing for better reconstruction [19]. Similarly, in parotid surgery, the exoscope allows easier identification of the facial nerve and its divisions, making the surgery safer and more comfortable [15, 20, 21].
Other uses of the exoscope
In addition to the previous indications, the 3D-exoscope is also used in our department for paediatric airway surgery, e.g., surgical treatment of laryngeal papillomatosis, laryngomalacia, laryngotracheal stenoses, external arytenoidopexy, or posterior laryngeal clefts. Alternating between endoscopy and exoscopy allows for good control of the procedure without having to modify the set-up (as opposed to alternating between microscopy and endoscopy). Similar results are found in the literature [14, 22].
Learning curve with the 3D-exoscope
Of note, significant incremental improvement in the score assigned to the contribution of the 3D-exoscope compared with usual practice over time was observed in our study (p < 0.001). The results are similar for cochlear implant surgeries where the operative technique is very standardized and similar from one operator to another. This shows that the use of the 3D-exoscope becomes a routine for the surgeon over time. Smith et al. also report increasing surgeon comfort as experience is gained [12].
Strengths and limitations of the study
This study has several strengths. First, it is the largest series of ENT surgeries performed with a 3D-exoscope and the first paediatric series to evaluate the different types of ENT surgeries (otologic surgery, transoral surgery, and head and neck surgery). Moreover, this prospective study reinforces the data in the literature concerning the growing interest of 3D-exoscopy in ENT, compared to our usual practices. This study also highlights the limits of this surgical tool, particularly in otology. Finally, this is the first study to show an improvement in exoscope use and surgeon comfort with time and experience.
The main limitation of this study is that it is not a comparative study to define if there is a real significant difference between the use of the optical aids or the 3D-exoscope, related to the fact that the results are based on the surgeon’s experience. Although the use of 3D-exoscopy in adult and now paediatric ENT is increasing, the current data in the literature are not sufficient for this method to replace microscopy. In addition, the data for transoral surgery and head and neck surgeries were evaluated against standard practice, which may be the use of a microscope or magnifying loupes depending on the surgeon. The performance of the exoscope necessarily exceeds the use of standard magnifying loupes and thus probably overestimates the evaluation of the interest of exoscopy, although it seems to be effective. In addition, our series did not report any cases of anterior skull base surgery (such as encephaloceles and nasal gliomas).
Conclusion
This large series of paediatric ENT surgeries supports the use of 3D-exoscopy, in several surgical fields, underlining the pedagogical advantages, easy participation of the surgical team, and involvement of the nursing and anaesthesia teams. The increasing use of 3D-exoscopy in ENT and neurosurgery demonstrates its surgical value. However, the 3D-exoscope used in this series (VITOM® 3D) has some limitations that could be corrected in future versions. Future comparative studies would be interesting to evaluate if microscopy can be replaced by 3D-exoscopy, subject to some technical improvements.
Data Availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Palumbo VD, Fazzotta S, Damiano G, Lo Monte AI (2018) VITOM® 3D system in surgeon microsurgical vascular training: our model and experience. J Vasc Access 19:108–109. https://doi.org/10.5301/jva.5000825
Rossini Z, Cardia A, Milani D et al (2017) VITOM 3D: preliminary experience in cranial surgery. World Neurosurg 107:663–668. https://doi.org/10.1016/j.wneu.2017.08.083
De Virgilio A, Mercante G, Gaino F et al (2020) Preliminary clinical experience with the 4 K3-dimensional microvideoscope (VITOM 3D) system for free flap head and neck reconstruction. Head Neck 42:138–140. https://doi.org/10.1002/hed.25979
Chebib E, Van Den Abbeele T, Benoit C (2021) Partial cricotracheal resection using a 3D-exoscopic visualization in children (with video). Eur Ann Otorhinolaryngol Head Neck Dis 138(Suppl 1):16–18. https://doi.org/10.1016/j.anorl.2021.02.017
Chebib E, Van Den Abbeele T, Benoit C (2021) Closure of a tracheo-esophageal fistula using a 3D-exoscopic visualization in a newborn (with video). Eur Ann Otorhinolaryngol Head Neck Dis 138(Suppl 1):10–11. https://doi.org/10.1016/j.anorl.2021.01.011
De Virgilio A, Festa BM, Costantino A et al (2021) High-definition 3D-exoscope-assisted soft palate excision and reconstruction. Head Neck. https://doi.org/10.1002/hed.26864
Bignami M, Arosio AD, Dalfino G et al (2021) First experience of ARTip Cruise VITOM-assisted OPF removal of frontal fibro-osseous lesion: operative video. Laryngoscope 131:2219–2223. https://doi.org/10.1002/lary.29546
Carobbio ALC, Filauro M, Parrinello G et al (2021) Microsurgical procedures during COVID-19 pandemic: the VITOM® 3D-HD exoscopic system as alternative to the operating microscope to properly use personal protective equipment (PPE). Eur Arch Otorhinolaryngol 278:2129–2132. https://doi.org/10.1007/s00405-020-06239-6
Colombo G, Ferreli F, Di Bari M et al (2021) Introducing the High-definition 3D-exoscope in ear surgery: preliminary analysis of advantages and limits compared with operative microscope. Eur Arch Otorhinolaryngol 278:4217–4223. https://doi.org/10.1007/s00405-020-06510-w
Rusetsky Y, Chuchueva N, Meytel I et al (2021) Exoscopic visualisation with VITOM® 3D in paediatric cochlear implantation: preliminary results. Clin Otolaryngol. https://doi.org/10.1111/coa.13902
Ferlito S, La Mantia I, Caruso S et al (2022) High definition three-dimensional exoscope (VITOM 3D) in E.N.T. surgery: a systematic review of current experience. J Clin Med 11:3639. https://doi.org/10.3390/jcm11133639
Smith S, Kozin ED, Kanumuri VV et al (2019) Initial experience with 3-dimensional exoscope-assisted transmastoid and lateral skull base surgery. Otolaryngol Head Neck Surg 160:364–367. https://doi.org/10.1177/0194599818816965
Rubini A, Di Gioia S, Marchioni D (2020) 3D exoscopic surgery of lateral skull base. Eur Arch Otorhinolaryngol 277:687–694. https://doi.org/10.1007/s00405-019-05736-7
Cantarella G, Pignataro L (2021) A high-definition 3-dimensional exoscope with the ARTip cruise system as an effective new tool for phonosurgery: a preliminary report. J Voice. https://doi.org/10.1016/j.jvoice.2021.07.008
Mincione A, Lepera D, Rizzi L (2021) VITOM 3D system in parotid gland surgery: our experience. J Craniofac Surg 32:e138–e141. https://doi.org/10.1097/SCS.0000000000006875
Wierzbicka M, Szyfter W, Greczka G, Gawęcki W (2021) Otosurgery with the high-definition three-dimensional (3D) exoscope: advantages and disadvantages. J Clin Med 10:777. https://doi.org/10.3390/jcm10040777
Minoda R, Miwa T (2019) Non-microscopic middle ear cholesteatoma surgery: a case report of a novel head-up approach. Otol Neurotol 40:777–781. https://doi.org/10.1097/MAO.0000000000002276
Crosetti E, Arrigoni G, Manca A et al (2020) 3D exoscopic surgery (3Des) for transoral oropharyngectomy. Front Oncol 10:16. https://doi.org/10.3389/fonc.2020.00016
Tasca I, Ceroni Compadretti G, Romano C (2016) High-definition video telescopic rhinoplasty. Acta Otorhinolaryngol Ital 36:496–498. https://doi.org/10.14639/0392-100X-1430
Bartkowiak E, Łuczewski Ł, Chou JT-T, Wierzbicka M (2021) Is the 3D-exoscope better than the surgical microscope in parotid surgery: a prospective, randomized single-center study. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-021-06876-5
Carta F, Mariani C, Marrosu V et al (2020) Three-dimensional, high-definition exoscopic parotidectomy: a valid alternative to magnified-assisted surgery. Br J Oral Maxillofac Surg. https://doi.org/10.1016/j.bjoms.2020.06.015
De Virgilio A, Costantino A, Mondello T et al (2021) Pre-clinical experience with the VITOM 3D and the ARTip cruise system for micro-laryngeal surgery. Laryngoscope 131:136–138. https://doi.org/10.1002/lary.28675
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by Chebib E. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical statement
The investigation adhered to the principles of the Declaration of Helsinki and was approved by our Institutional Human Experimentation Committee (Institutional Review Board 00006477).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 56579 KB)
Supplementary file3 (MP4 67456 KB)
Supplementary file4 (MP4 49581 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chebib, E., Benoit, C., Bois, E. et al. New surgical frontiers for 4K 3D-exoscope in paediatric head and neck surgery. Eur Arch Otorhinolaryngol 280, 2033–2041 (2023). https://doi.org/10.1007/s00405-022-07785-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-022-07785-x