Abstract
Purpose
The aim of this study was to analyze the hearing outcomes and quality of life in a series of 52 patients affected by conductive or mixed hearing loss and treated with Bonebridge®.
Methods
52 of 71 patients implanted with Bonebridge® between October 2012 and January 2022, were included in the study. We compared the air conduction thresholds at the frequencies 500, 1000, 2000, 3000, 4000 Hz, the SRT50% and the World Recognition Score at an intensity of 50 dB with and without the implant. The Abbreviated Profile of Hearing Aid Benefit (APHAB) was employed to assess the quality of life of patients.
Results
The liminal tone audiometry (free field) pure tone average for air conduction after 6 months with the implant was 35.12 dB, obtaining a mean gain of 31.83 dB. With Bonebridge®, the mean SRT was 34.17 dB, whereas before the surgery no patient achieved 50% of correct answers at a sound intensity of 50 dB. The world recognition score at 50 dB changed from 11% without the implant to 85% with it. We observed one case of implant failure and one case of implant exposure. The APHAB questionnaire showed an improvement after implantation in practically all the subscales.
Conclusions
The hearing outcomes and the subjective benefits reported by patients obtained in our study are similar to those published in the literature. Bonebridge® represents an excellent method for the rehabilitation of patients with conductive and mixed hearing loss, showing a low rate of complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone conduction devices (BCDs) are an established form of treatment for conductive and mixed hearing loss as well as single-sided sensorineural deafness (SSD) [1].
In 1977, Tjellström et al. were the first clinicians to insert a bone conduction hearing implant [2]. Now, 45 years later, more than 150,000 individuals have achieved auditory rehabilitation with bone conducting hearing implants.
BCDs work by converting sound energy into vibration of the skull bones. The sound is transmitted through the skull bone, cartilage, skin and soft tissue, and fluids in the body, ultimately resulting in a sound pressure in the basilar membrane [1].
There are different types of bone conduction devices that may be classified according to the mode of conduction [3].
-
Cutaneous: the vibration is transmitted through the skin (passive transcutaneous, for example, Sophono, BAHA® [Bone Anchored Hearing Aid, Cochlear Co., Australia] attract)
-
Direct: the vibration is transmitted directly to the bone without passing through the skin. These are divided into percutaneous (BAHA®, Ponto), and active transcutaneous (Bonebridge® [Vibrant MED-EL, Innsbruck, Austria], Cochlear Osia OSI200 Implant)
The Bonebridge® device BCI 601 was first implanted in June 2011 as part of a clinical trial and was launched onto the EU market in September 2012 [4].
Initially, it was approved for patients over 18 years of age and in 2014 for those over 5 years of age [5].
Bonebridge® is a partially implantable device that consists of two components. One component is external and includes an audio processor with two microphones for receiving sounds in the environment, a digital compression processor, and a battery. The other internal component or Bone Conduction Implant (BCI) system involves a magnet surrounded by a receptor wheel, an electronic device (demodulator), a transition, and an electromagnetic BCI 601 (BC-FMT) floating mass transducer that is surgically implanted into the skull in either the transmastoid, retrosigmoid, or middle fossa regions [6, 7]. The implant is inserted completely below the skin, connected to a processor through magnetic attraction, and transforms the signal received into mechanical vibrations that are transmitted through the temporal bone to the inner ear.
In September 2019, the second generation of Bonebridge®, the Bonebridge® BCI 602 was introduced. The most significant difference from the BCI 601 (first generation) is the shape and size of the internal part, such that the thickness of the BC-FMT decreased from 8.7 to 4.5 mm, with nearly 50% less drilling depth. This feature—together with great flexibility of the implant and the use of self-drilling screws—simplifies handling, thereby significantly reducing surgical time and the risk of dural exposure [8,9,10].
Unlike transcutaneous devices for signal transmission, osseointegration of the cortical fixation screws is not crucial [5], resulting in a lower complication rate than percutaneous systems and higher and more reliable hearing gain compared to other transcutaneous or percutaneous systems [4, 5]. Furthermore, the fast activation of the implant system enables the recipient of the system to benefit postoperatively from the intervention in a short time frame.
Audiological criteria [5]
Audiological criteria for the Bonebridge® implant recommended by MED-EL are (Fig. 1):
-
1.
Conductive or mixed mild-to-moderate hearing loss; pure tone average (PTA) bone conduction (BC) thresholds (measured at 0.5, 1, 2, 3, and 4 kHz) > = 45 dB HL; any central or retrocochlear disorder must be ruled out
-
2.
Profound sensorineural hearing loss in one ear and normal hearing in the opposite ear and air conduction hearing thresholds of the hearing ear > = 20 dBHL (measured at 0.5, 1, 2, 3, and 4 kHz)
In addition to audiological criteria, a detailed personal medical history must be performed to rule out certain medical conditions:
-
Previous tympanoplasty, external ear canal stenosis or chronic infections that make the use of conventional hearing aids impossible
-
Otosclerosis or tympanosclerosis that cannot be treated with surgery or conventional hearing aids
-
Sudden hearing loss, vestibular Schwannoma, or other causes of single-sided deafness
-
Unfavorable temporal bone anatomy, previously valued by CT scan, to ensure safe placement of the implant
-
Retrocochlear or central disorder
-
Psychological/psychiatric disorders
The aim of this study was to assess the effectiveness of Bonebridge® in a large series of 52 patients diagnosed with conductive/mixed hearing loss, reporting the audiological outcomes and subjective benefits in hearing-related quality of life (QoL). A secondary goal was to analyze the complication rate.
Materials and methods
A retrospective study was made of a series of 71 patients who had received treatment with the Bonebridge® system between October 2012 and January 2022 in the Otorhinolaryngology and Head and Neck Surgery Department of the Son Espases University Hospital, Palma, Spain. The aim of the study was to analyze the audiological outcomes obtained in patients diagnosed with conductive/mixed hearing loss, the complication rate, and the subjective benefits in hearing-related QoL.
Audiological evaluation
A pre- and postoperative audiological analysis was carried out in all patients. This included a liminal tone audiometry (free field) and a speech audiometry with contralateral masking. Minimum follow-up was 6 months.
Inclusion criteria for audiological analysis.
-
Conductive and mixed bilateral hearing loss with thresholds in bone conduction greater than or equal to 45 dB in frequencies of 0.5, 1, 2, 3, 4 kHz
-
Patients older than 5 years
-
Minimum follow-up of 6 months
Exclusion criteria for audiological analysis.
-
Patients diagnosed with severe/profound unilateral hearing loss
Of 71 patients, 19 cases of severe/profound single-sided sensorineural hearing loss were excluded from audiological analysis. Finally, 52 patients met the inclusion criteria and were included in the study. All patients were preoperatively subjected to a Computed Tomography with contrast to assess the best approach for the safest placement of the device. First generation of Bonebridge® (BCI 601) was implanted from October 2012 to August 2019. From September 2019 the second generation of Bonebridge® (BCI 602) was implanted in 26 patients, 15 of whom had been diagnosed with conductive or mixed hearing loss and included in the study.
To choose which side to implant, all patients underwent a bandwidth test, through observing the benefits in tone and speech audiometry and subjective perception of well-being reported by the patient. An audiological evaluation in the pre and postoperative setting was performed, by analyzing air conduction (free field in the postoperative analysis) and bone conduction thresholds in the liminal tone audiometry as well as the discrimination thresholds in the speech audiometry. The pure tone average (PTA) based on the frequencies of 0.5, 1, 2, and 4 kHz was calculated for the air and bone conduction prior to the surgery, and its value was compared with the free field air conduction PTA with the use of the device. With regard to the speech audiometry, two parameters were calculated in the pre- and postoperative setting: (1) the Speech Recognition Threshold 50% (SRT50), defined as the intensity in dB at which the patient achieves 50% discrimination; and (2) The Word Recognition Score (WRS), which is the percentage of correct answers at an intensity of 50 dB.
Quality of life
The Abbreviated Profile of Hearing Aid Benefit (APHAB) was employed to assess the QoL of patients implanted with Bonebridge®. This is a 24-item inventory that assesses the amount of difficulty that a person experiences when communicating in a variety of conditions and enables pre- and post-operative results to be compared in the same session. The APHAB provides a global score as well as subscale scores for ease of communication, reverberation, background noise, and aversiveness domains. As reported by Magele et al. [4] in their recent meta-analysis, APHAB stands out as the most frequently used tool to assess hearing-related QoL benefits in patients with Bonebridge®.
Complication rate
Minor complications (with no need for revision surgery), major complications (requiring revision surgery), and cases of failure of the implant were analyzed in all patients.
Results
A total of 52 patients were included in the study (Table 1), 28 women and 24 men with a ratio of 1.16–1 aged between 19 and 74 years (mean age 50.205 years). Thirty-one devices were implanted in the right ear and 21 in the left ear, all unilaterally. Regarding medical history, eight patients had previously had surgery for bilateral cholesteatomatous otitis media; 31 for chronic bilateral otitis media; three for recurrent external otitis, which prevented the use of standard hearing aids; one for aural agenesia; nine for otosclerosis, from which five had not improved with stapedotomy surgery; one for whom the surgery could not be completed, due to the persistence of the stapedial artery which obliterated the oval window, while three refused surgical treatment. From September 2019, the second generation of Bonebridge® (BCI 602) was implanted in 26 patients, 15 of whom had been diagnosed with conductive or mixed hearing loss and were included in the study. The middle fossa approach and presigmoid approach were employed in 11 and four patients, respectively.
Activation of the devices was always planned 3 weeks after surgery. In all cases, patients were visited 10 days after the intervention to remove the sutures. Mean surgical time was 47.8 min. A presigmoid, retrosigmoid, and middle fossa approach were employed in 23, 4, and 25 patients, respectively.
Audiological results
Tone audiometry comparison
All patients met the Bonebridge® audiological criteria of bone conduction thresholds (less than or equal to 45 dB HL); the mean preoperative bone conduction thresholds were 24, 25, 39, 39, and 38 for the frequencies of 0.5, 1, 2, 3, 4 kHz, respectively. The preoperative free field air conduction thresholds were 68.4 dB at 500 Hz; 64.32 dB at 1000 Hz; 64.26 dB at 2000 Hz; 68.9 dB at 3000 Hz; and 70.84 dB at 4000 Hz.
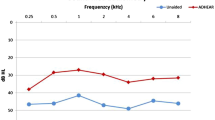
The audiological outcomes with the use of the implant in free field were 39.2 dB at 500 Hz; 30.4 dB at 1000 Hz; 32.76 dB at 2000 Hz; 32.85 dB at 3000 Hz; and 38.12 dB at 4.000 Hz (Fig. 2).
The functional gain in dB was 29.2, 33.92, 31.5, 36.05, and 32.72 dB in the respective frequencies.
Pure tone average or PTA for bone conduction were 31.5 dB. The liminal tone audiometry (free field) PTA for air conduction was 66.9 dB. After 6 months, the liminal tone audiometry (free field) PTA with a functioning device was 35.12 dB, obtaining a mean gain of 31.83 dB (Fig. 3).
Speech audiometry comparison
In relation to the results of the speech audiometry, two parameters were studied:
-
SRT50 up to an intensity of 50 dB
-
Percentage of correct answers at an intensity of 50 dB or Word Recognition Score at 50 dB (WRS50dB)
With Bonebridge®, the mean SRT was 34.17 dB, while without Bonebridge® practically none of the patients obtained 50% of correct answers to the assessed intensities (up to 50 dB), except for one who accomplished it at 50 dB. This finding indicates that when patients use the Bonebridge® system they are able to understand half of the words at a mean of 34.17 dB while masking the contralateral ear, contrary to what happens without it, in which case only one was able to complete the test successfully.
When the percentage of correct answers was evaluated at 50 dB (WRS50dB), the patients with the implant obtained 85% of correct answers, whereas those without it only obtained 11% (Fig. 4).
Complication rate
No adverse events were observed during surgeries and no complications were noted in the immediate postoperative period.
One case of implant exposure was observed in a patient with a previous face lift [11]. In this case the Bonebridge® had been implanted in the retroauricular region using a classic presigmoid approach. After oral antibiotic treatment, surgical debridement was performed, and a rotational skin flap was needed to close the defect. In the end, the same device was implanted at the level of the middle fossa on the squamous portion of the temporal bone. In another patient with chronic otitis media with effusion, poor performance of the implant was observed. Hence, the implant was removed from the presigmoid region and implanted at the level of the middle fossa on the squamous portion of the temporal bone, with a satisfactory result. Finally, one case of implant failure was found in a patient in whom the Bonebridge® had been placed in the retrosigmoid region. In this case, the implant was removed, and another device was placed at the level of the middle fossa on the squamous portion of the temporal bone.
No major adverse events (meningitis, empyema, or lateral sinus thrombosis) were observed in our series during the follow-up (6–116 months).
Quality of life. Results
Patient-reported outcomes
The APHAB questionnaire showed an increase in QoL after implantation of the Bonebridge®. Global scores decreased significantly from 56% (SD 10.4%) before surgery to 26.2% (SD 8.2%) after 6 months of follow-up (Fig. 5; Paired t test p < 0.01). Similarly, significant improvements were observed in background noise (mean change 34.1; 95% CI 31–37.2), reverberation (mean change 22.77; 95% CI 19.85–25.7), and ease of communication (mean change 32.15; 95% CI 28.4–35.8) subscales. In the aversiveness subscale, no significant changes were found (mean change 1.17; 95% CI 0.1–2.8) (Fig. 5).
Results of the APHAB questionnaire. EC Ease of Communication, RV reverberation, BN background noise, AV aversiveness, GS global score. The differences in the EC, BN, and RV subscales are statistically significant at p < 0.01, but the difference in the AV subscale is not. Bars represent mean scores; error bars represent standard deviation
Discussion
The audiological outcomes observed in our series confirm the effectiveness of the Bonebridge® in patients with conductive/mixed hearing loss, revealing similar results to those observed in a previous series published by Carnevale et al. [3].
A significant improvement in air conduction thresholds in liminal tone audiometry and in speech discrimination is observed. PTA for liminal tone audiometry (free field) improved from a preoperative value of 66.9 to 35.12 dB 6 months after surgery with a functioning device, obtaining a mean functional gain of 31.83 dB. Our results confirm the data observed in other studies, where functional gain was reported from 24 to 37 dB, and in 50% of cases better than 30 dB [5, 6, 12].
Regarding speech audiometry, the word recognition score at 50 dB was 85% with Bonebridge® (only 11% before surgery) and the mean SRT50 (50% speech recognition threshold) was 34.17 dB with Bonebridge® 6 months after surgery, while without Bonebridge® practically none of the patients obtained 50% of correct answers in the assessed intensities (up to 50 dB). These results at 6 months after surgery match with the data observed in the first European multicenter study published by Sprinzl et al. [6], in which a gradual improvement in SRT50 was observed during the first few months after surgery, with an SRT50 of 36.6 dB at 3 month follow-up.
Similar findings were observed by Skarżyński et al. [13], with WRS improving from 0, 43, and 62% before surgery to 40, 74, and 87% after 3 months and 43, 75, and 89% after 6 months of follow-up, respectively, in all three level settings (50, 60, 70 dB).
A significant improvement in speech recognition over time was also observed by Seiwerth et al. [14] in a recent article published in 2022, where word recognition score in quiet at 65 dB SPL improved from 11% preoperatively to 74% at 3 months and 83% at > 11 months.
This effect was also reported in children by Baumgartner et al. [15] who suggested device acclimation effects and additional fitting procedures as possible factors. For this reason, the authors report the hearing outcomes obtained at 6 months of follow-up.
Regarding patient-reported outcome measures, the benefits in patient satisfaction are in line with hearing benefits measured by functional gain, and WRS. The APHAB questionnaire is considered to be a very good tool for the assessment of hearing-related QoL in Bonebridge® patients, as it is easy to use and enables pre- and postoperative performances to be compared during the same session. A significant improvement was observed in all domains except for the aversiveness subscale, which showed no significant changes. This observation is similar to other studies, in which the AV subscale did not change significantly or was prone to increase [13, 16, 17].
From the surgical point of view, the Bonebridge® can be implanted in three different locations [18]. In cases of normal anatomy demonstrated on the pre-operative CT scan, it is placed in the sinodural angle, in a presigmoid location. If the mastoid is already drilled out or if there is little space in the sinodural angle, the bone conduction floating mass transducer can be placed either behind the sinus [19, 20] (retrosigmoid approach) or above the temporal line in the middle fossa region [21].
In our department, when an anatomical variant exists, such as a low middle fossa dura plate or anterior procident sigmoid sinus, or previous surgery such as a petrosectomy or a radical mastoid cavity has been performed, we prefer the middle fossa approach, placing the bone conduction floating mass transducer on the squamous portion of the temporal bone so as to avoid exploring the previously operated ear. In 2014 the authors stopped performing the retrosigmoid approach and 5 years later published an article describing their preferred technique for the middle fossa approach with the first generation of Bonebridge®, using a 14 mm drill head (Neuro Drill) to create the bed at the squamous portion of the temporal bone [22].
The authors observed that the use of the Neuro Drill avoided the risk of damage to the dura mater observed with the classic otologic drill, since its mechanism of action is based on the automatic interruption of its movement when it does not find bone resistance, which guarantees the integrity of the dura mater. When compared with the retrosigmoid approach, this technique turned out to be easier, surgical time was significantly shorter, and there was no risk of damaging the sigmoid sinus or dura mater, which are the main risks of the retrosigmoid approach. In September 2019, with the introduction of the second generation of the implant, we stopped using the neuro drill for the middle fossa approach.
Indeed, the change in size and shape of the internal part, with a reduced thickness of the BCM–FMT from 8.7 to 4.5 mm enables nearly 50% less drilling depth, thereby reducing drilling time and the likelihood of dural exposure. This is a considerable advantage and gives opportunities for using it on patients, where it was impossible to use the first-generation implant due to limited anatomical conditions [8].
In addition to the reduced size and less drilling depth, the second generation of Bonebridge® affords several advantages that make the surgical procedure safer and faster. The first one is its flexible surgical placement. In fact, the flexible transition between the receiver coil and the floating mass transducer enables it to bend up to 90° in either lateral direction and medially up to 30° to accommodate the curvature of the skull. In addition, self-drilling screws simplify handling [23, 24].
In this series the presigmoid, retrosigmoid, and middle fossa media approaches were used in 23, 4, and 25 patients, respectively, and the second generation of Bonebridge® was implanted in 15 of 52 cases, 11 of which were in the middle fossa and 4 in the presigmoid region.
Safety of Bonebridge® has been well-documented in the literature [3, 18, 25,26,27,28,29]. In our series, no minor complications, such as skin infection, edema, or local pain, were reported. One major complication with skin necrosis and extrusion of the implant in a patient with previous facelift surgery was observed, resulting in a 1.9% rate of major complications. Furthermore, one case of implant technical failure and one case of poor performance of the implant, because recurrent middle ear effusion was observed. Similar data have recently been published by Magele et al. [4] and Sprinzl et al. [5] with a major complication rate of 0.85% and 1.7%, respectively.
Existence of previous cervicofacial surgery is a recognized risk factor for implant extrusion, since it may compromise the vascularization of the retroauricular region and cause dehiscence and necrosis of the skin. In the event of previous cervicofacial surgery, where postauricular vascularization depending on the posterior auricular artery can be compromised, we propose the following: 1) harvest two flaps: a superficial flap that includes the skin and subcutaneous tissue; and a deeper fascio-muscular flap, to reduce the risk of device extrusion; 2) consider the middle fossa approach the best option to avoid a large postauricular incision in the conflictive area [20, 30].
Conclusions
The results observed in this series reveal a significant improvement in hearing and speech discrimination with a low rate of complications. The subjective benefits reported by patients are in line with audiological outcomes, with a significant improvement in quality of life. This surgery is safe and reproducible, nonetheless the most adequate surgical approach must be evaluated preoperatively to reduce the risk of adverse events. In conclusion, our study confirms that the Bonebridge® is a very effective and safe method for the audiological rehabilitation of patients with conductive/mixed hearing loss.
References
Stenfelt S (2011) Acoustic and physiologic aspects of bone conduction hearing. Adv Otorhinolaryngol 71:10–21
Tjellström A, Rosenhall U, Lindstrom J, Hallen O, Albrektsson T, Branemark I (1983) Five-year experience with skin-penetrating bone-anchored implants in the temporal bone. Acta Otolaryngol 95:568–575
Carnevale C, Til-Pérez G, Arancibia-Tagle DJ, Tomás-Barberán MD, Sarría-Echegaray PL (2019) Hearing outcomes of the active bone conduction system Bonebridge ® in conductive or mixed hearing loss. Acta Otorrinolaringol Esp 70(2):80–88
Magele A, Schoerg P, Stanek B, Gradl B, Sprinzl GM (2019) Active transcutaneous bone conduction hearing implants: systematic review and meta-analysis. PLoS ONE. https://doi.org/10.1371/journal.pone.0221484
Sprinzl GM, Wolf-Magele A (2016) The bonebridge bone conduction hearing implant: Indication criteria, surgery and a systematic review of the literature. Clin Otolaryngol 41:131–143
Sprinzl G, Lenarz T, Ernst A, Hagen R (2013) First European Multicenter results with a new transcutaneous bone conduction hearing implant system: short term safety and efficacy. Otol Neurotol 34:1076–1083
Manrique M, Sanhueza I, Manrique R, de Abajo J (2014) A new bone conduction implant: surgical technique and results. Otol Neurotol 35:216–220
Cywka KB, Skarzynski H, Król B, Skarzynski PH (2021) The Bonebridge BCI 602 active transcutaneous bone conduction implant in children: objective and subjective benefits. J Clin Med 10(24):5916. https://doi.org/10.3390/jcm10245916
Plontke SK, Götze G, Wenzel C, Rahne T, Mlynski R (2020) Implantation of a new active bone conduction hearing device 395 with optimized geometry. HNO 68:106–115. https://doi.org/10.1007/s00106-020-00877-2.396
Utrilla C, Gavilán J, García-Raya P, Calvino M, Lassaletta L (2020) MRI after Bonebridge implantation: a comparison of two 397 implant generations. Eur Arch Otorhinolaryngol 278:3203–3209. https://doi.org/10.1007/s00405-020-06380-2
Carnevale C, Til-Pérez G, Arancibia-Tagle D, Tomás-Barberán M, Sarría-Echegaray P (2021) Cervicofacial surgery and implantable hearing device extrusion: management of challenging cases. J Laryngol Otol 135(3):212–216
Rivas JA, Rincón LA, Garcia L, Rivas A, Tamayo C, Forero VH (2013) Implantes auditivos de conducción ósea percutáneo, transcutáneo: comparación. Acta Otorrinolaringol Cir Cabeza Cuello 41:17–24
Skarżyński PH, Ratuszniak A, Król B, Kozieł M, Osińska K, Cywka KB, Sztabnicka A, Skarżyński H (2019) The Bonebridge in adults with mixed and conductive hearing loss: audiological and quality of life outcomes. Audiol Neurotol 24:90–99
Seiwerth I, Fröhlich L, Schilde S, Götze G, Plontke SK, Rahne T (2022) Clinical and functional results after implantation of the bonebridge, a semi-implantable, active transcutaneous bone conduction device, in children and adults. Eur Arch Otorhinolaryngol 279(1):101–113
Baumgartner WD, Hamzavi JS, Boheim K, Wolf-Magele A, Schlogel M, Riechelmann H, Zorowka P, Koci V, Keck T, Potzinger P, Sprinzl G (2016) A new transcutaneous bone conduction hearing implant: short-term safety and efficacy in children. Otol Neurotol 37(6):713–720. https://doi.org/10.1097/MAO.0000000000001038
Schmerber S, Deguine O, Marx M, Van de Heyning P, Sterkers O, Mosnier I, Garin P, Godey B, Vincent C, Venail F, Mondain M, Deveze A, Lavieille JP, Karkas A (2017) Safety and effectiveness of the Bonebridge transcutaneous active direct-drive bone-conduction hearing implant at 1-year device use. Eur Arch Otorhinolaryngol 274(4):1835–1851
Garciera M, Lavedrinea A, Gagneuxa C, Eluecquec T, Grayeli AB (2021) Bone-anchored and closed skin Bonebridge implant in adults: hearing performances and quality of life. Audiol Neurootol 26(5):310–316. https://doi.org/10.1159/000512496
Barbara M, Perotti M, Gioia B, Volpini L, Monini S (2013) Transcutaneous bone-conduction hearing device: audiological and surgical aspects in a first series of patients with mixed hearing loss. Acta Otolaryngol 133:1058–1064
Zernotti ME, di Gregorio MF, Galeazzi P, Tabernero P (2016) Comparative outcomes of active and passive hearing devices by transcutaneous bone conduction. Acta Otolaryngol 136:556–558
Lassaletta L, Sanchez-Cuadrado I, Muñoz E, Gavilan J (2014) Retrosigmoid implantation of an active bone conduction stimulator in a patient with chronic otitis media. Auris Nasus Larynx 41:84–87
Zernotti ME, Sarasty AB (2015) Active bone conduction prosthesis: Bonebridge (TM). Int Arch Otorhinolaryngol 19:343–348
Carnevale C, Tomás-Barberán M, Til-Pérez G, Sarría-Echegaray P (2019) The Bonebridge active bone conduction system: a fast and safe technique for a middle fossa approach. J Laryngol Otol 133(4):344–347
Plontke SK, Götze G, Wenzel C, Rahne T, Mlynski R (2020) Implantation of a new active bone conduction hearing device with optimized geometry. HNO 68:106–115
You P, Siegel LH, Kassam Z, Hebb M, Parnes L, Ladak HM, Agrawal SK (2019) The middle fossa approach with self-drilling screws: a novel technique for BONEBRIDGE implantation. J Otolaryngol Head Neck Surg 48:1–10
Lassaletta L, Calvino M, Zernotti M, Gavilan J (2016) Postoperative pain in patients undergoing a transcutaneous active bone conduction implant (Bonebridge). Eur Arch Otorhinolaryngol 273(12):4103–4110
Law EK, Bhatia KS, Tsang WS, Tong MC, Shi L (2016) CT pre-operative planning of a new semi-implantable bone conduction hearing device. Eur Radiol 26(6):1686–1695. https://doi.org/10.1007/s00330-015-3983-x
Bianchin G, Bonali M, Russo M, Tribi L (2015) Active bone conduction system: outcomes with the Bonebridge transcutaneous device. ORL J Otorhinolaryngol Relat Spec 77(1):17–26. https://doi.org/10.1159/000371425
Weiss R, Leinung M, Baumann U, Weissgerber T, Rader T, Stover T (2017) Improvement of speech perception in quiet and in noise without decreasing localization abilities with the bone conduction device Bonebridge. Eur Arch Otorhinolaryngol 274(5):2107–2115. https://doi.org/10.1007/s00405-016-4434-2
Wimmer W, Gerber N, Guignard J, Dubach P, Kompis M, Weber S, Caversaccio M (2015) Topographic bone thickness maps for Bonebridge implantations. Eur Arch Otorhinolaryngol 272(7):1651–1658. https://doi.org/10.1007/s00405-014-2976-8
David T, Ramsden JD, Gordon KA, James AL, Papsin BC (2009) Soft tissue com- plications after small incision pediatric cochlear implantation. Laryngoscope 119:980–983
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors have provided substantial contributions to the conception or design of the work or the interpretation of data for the work. All of them worked on the draft or revised it critically for important intellectual content. The final version was approved for publishing by all authors. The authors agree on accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The work was approved by ethics committee of Son Espases University Hospital and was performed in accordance with the ethical standards.
Consent for publication
All authors give have seen this manuscript and agree with its contents for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carnevale, C., Morales-Olavarría, C., Til-Pérez, G. et al. Bonebridge® bone conduction implant. Hearing outcomes and quality of life in patients with conductive/mixed hearing loss. Eur Arch Otorhinolaryngol 280, 1611–1619 (2023). https://doi.org/10.1007/s00405-022-07631-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-022-07631-0