Abstract
Purpose
Evidence suggests that patients’ skeletal muscle mass (SMM) can predict the patients at risk for cisplatin dose-limiting toxicities (DLT). Cisplatin is currently dosed on body surface area (BSA). The predictive value of SMM for cisplatin DLT in patients with locally advanced head and neck cancer (LA-HNC) is investigated.
Methods
Patients with LA-HNC treated with cisplatin-based chemoradiotherapy (CRT) were included. SMM was measured using pre-treatment scans. Logistic regression analysis was performed to identify the predictive impact of low SMM for DLT.
Results
In total, 343 patients were included of which 199 patients (58.0%) had low SMM and 154 patients (44.9%) experienced cisplatin DLT. In multivariate analysis, low SMM at diagnosis was the only predictive factor for DLT (HR 1.8, 95% CI 1.1–2.9).
Conclusions
Low SMM was associated with an increased risk of DLT. Trials are needed to investigate cisplatin dosing with consideration of SMM rather than solely BSA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer accounts worldwide for more than 500,000 cases annually [1]. Locally advanced head and neck cancer (LA-HNC) is the most frequent clinical manifestation of head and neck cancer. Platinum-based chemoradiotherapy is the main treatment option for (technical or functional) irresectable LA-HNC and is also offered in a postoperative setting for resected LA-HNC in which the tumor is resected with positive margins or in the presence of extracapsular lymph-nodal extension.
Malnutrition is a common problem in LA-HNC in part due to dysphagia caused by the tumor or its treatment [2]. Malnutrition is also a major contributor to the development of low skeletal muscle mass (SMM). Image-based analysis of SMM has shown critical new insights of low SMM as an important predictor and prognosticator in patients with cancer [3,4,5]. Cisplatin is the preferred platinum agent used in platinum-based chemoradiotherapy in LA-HNC. Cisplatin is dosed based on body surface area. This approach was initially advocated on the assumption that dosing based on body surface area leads to an acceptable degree of toxicities without reducing the therapeutic effect [6]. Cisplatin is highly emetogenic, neurotoxic, nephrotoxic and ototoxic [7]. Clinically, there is a wide interindividual heterogeneity in the ability of LA-HNC patients to tolerate cisplatin-based chemoradiotherapy. Over the last years, emerging evidence suggests a significant negative relationship between low SMM and adverse effects of cytotoxic drugs leading to dose-limiting toxicities (DLTs) [8,9,10,11]. As such, SMM may (partly) explain the heterogeneity of patient’s tolerance for chemotherapy. Cisplatin DLTs lead to frequent hospital readmissions, decreased survival and reduced quality of life.
The mechanism underlying the relationship between low SMM and DLTs of chemotherapeutical drugs is not fully understood. Several hypotheses have been proposed in the literature [12, 13]. It has been hypothesized that altered fat-to-lean body mass (LBM) influences the pharmacokinetics of anti-cancer drugs and/or may be associated with increased chronic low-grade inflammation, which results in a higher risk of adverse events. The most commonly supported hypothesis is based on the influence of low SMM on the volume of distribution of anti-cancer drugs [13].
Cisplatin is a hydrophilic agent; due to its hydrophilicity it favors distribution to the LBM of which SMM is the largest component. However, SMM is currently not (directly) taken into account in cisplatin dosing. Body surface area is calculated by use of several formulas such as the formula of Du Bois [6]. These formulas incorporate body weight and height. Lower SMM can, however, occurs independently of adiposity, therefore in overweight or obese patients, the loss of SMM may be masked. Hence, dosing according to body surface area leads to substantial variation in drug doses per kilogram of LBM [8]. Higher dose per kilogram LBM has shown to have a significant correlation with higher rates of toxicities in other cancer types [9]. A loss of SMM in patients with head and neck cancer may, consequently, induce drug overdose when dose calculation is based on the conventional body surface area method.
In our previous study, we found a predictive impact of low SMM for platin (carboplatin and cisplatin) DLT. [8] As mentioned previously, cisplatin is the preferred platinum agent used in the treatment of LA-HNC. Cisplatin-unfit patients receive carboplatin, these patients are, however, more likely to have low SMM due to their comorbidities.
Therefore, the aim of the current study was to investigate the predictive impact of low SMM for cisplatin DLT in a 10 year cohort of patients with LA-HNC treated with cisplatin-based chemoradiotherapy. Patients who received cisplatin in our previous study are also included in this study.
Materials and methods
Study design
A retrospective study was conducted in which all patients who were diagnosed with LA-HNC and treated in the UMC Utrecht with cisplatin-based chemoradiotherapy between 2007 and 2018 were screened for inclusion. Inclusion criteria for this study required that patients were treated with curative intent in primary or adjuvant setting and had pre-treatment imaging of the head and neck area within 1 month before the start of chemoradiotherapy and had data available on cisplatin dosages and reported toxicities. Relevant demographic, clinical, biochemical and anthropometric variables were retrieved from electronic medical records. This study also included the patients who were treated with cisplatin from our previous study [8].
Ethical approval
The design of this study was approved by the Medical Ethical Research Committee (METC) of the University Medical Center Utrecht, METC ID: 17-365/C. The requirement for informed consent from patients was waived because of its retrospective design.
Therapy
Chemotherapy regimen consisted of three cycles of intravenous cisplatin-based chemotherapy on days 1, 22 and 43 of treatment. Cisplatin dose was 100 mg per m2 of body surface area. Chemoradiotherapy was given in the primary setting for patients with (technical or functional) irresectable LA-HNC and in postoperative setting for tumors with their aforementioned high-risk features. Radiotherapy was administered in 35 fractions of 2 Gy to a total dose of 70 Gy (primary setting) or in 33 fractions of 2 Gy to a total dose of 66 Gy (postoperative setting).
Body composition measurements—skeletal muscle mass and lean body mass
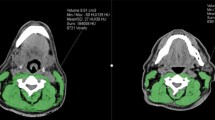
SMM was segmented as skeletal muscle area using the Slice-O-matic software (version 5.0). At the level of the third cervical vertebrae (C3), a single slice was used for skeletal muscle area segmentation. The first slide to completely show the entire vertebral arc when scrolling through the C3 vertebra from caudal to cephalic direction was selected. For computed tomography (CT) imaging, muscle area was defined as the pixel area between the radiodensity range of -29 and +150 hounsfield units, which is specific for muscle tissue [14]. For magnetic resonance imaging (MRI), muscle area was manually segmented, and fatty tissue was manually excluded. Because the overall intraclass correlation coefficient for the skeletal muscle area obtained by CT and MRI was previously found to be excellent (ICC 0.97, p < 0.01) [15], skeletal muscle area measurements by CT and MRI were analyzed together. Figure 1. shows an example of skeletal muscle segmentation at the level of C3. The skeletal muscle area was calculated as the sum of the delineated areas of the paravertebral muscles and both sternocleidomastoid muscles. In cases of infiltration of the sternocleidomastoid muscle, we doubled the single skeletal muscle area of the not infiltrated sternocleidomastoid muscle. This in accordance with our previous study of Swartz et al. in which we found that this method was equally predictive for skeletal muscle area of SMM at L3 in comparison with the paravertebral muscles and both available sternocleidomastoid muscles [16].
SMM is often used interchangeably with LBM, however, LBM includes SMM, as well as bones and bodily fluids. Therefore, we also predicted LBM using Mourtzakis formula where the LBM in kilograms by use of the skeletal muscle area obtained by cross-sectional imaging was used [17]. Mourtzakis formula is based on skeletal muscle area measured at the third lumbar vertebrae (L3), not C3. Therefore, skeletal muscle area at the level of C3 was first converted to skeletal muscle area at the level of L3 using a previously published formula [16].
Absolute SMM is strongly correlated with height, therefore SMM must be calculated as an index of relative SMM [18]. This is the same as the use of body mass index [body weight (kg)/height2 (m2)] for classifying relative adiposity. Skeletal muscle area at L3 is normalized to stature (using squared height similar to calculating BMI) to obtain the lumbar skeletal muscle mass index (LSMI).
The LSMI cut-off value used in this study was a LSMI of 43.2 cm2/m2, as previously established in a separate cohort of LA-HNC patients [8]. This cut-off value was used to categorize patients into patients with low SMM and patients without low SMM. Thus, in further analysis low SMM was defined as LSMI ≤ 43.2 cm2/m2.
Dose-limiting toxicity
We defined cisplatin DLT as any toxicity resulting in a cisplatin dose-reduction of ≥ 50%, a treatment delay of ≥ 4 days or a termination of cisplatin-based chemotherapy after the first or second cycle of therapy.
Statistical analysis
Data analysis was performed using IBM SPSS statistics 25. Demographic, clinical, biochemical and anthropometric data were reported for the total group and according to SMM and DLT status. Baseline measures for these groups were described using descriptive statistics. Normally distributed variables were shown as means ± standard deviation (SD), non-normally distributed variables were shown as medians with an interquartile range (IQR). Normality was investigated using the Kolmogorov–Smirnov test. Categorical variables were described as frequencies with corresponding percentages. Chi-square statistics were used for analyzing differences between the frequencies of each categorical variable with the presence or absence of low SMM and DLT.
Wald logistic regression analysis was used for univariate and multivariate analysis of the predictors for cisplatin DLT. Covariates used in the multivariate analysis were selected based on statistical significance in univariate analysis or on clinical relevance. Statistical significance was evaluated at the 0.05 level using 2-tailed tests. The Hosmer–Lemeshow test was performed to test the goodness-of-fit of the multivariate analysis model.
Results
Patient characteristics
In total, 343 patients were included between January 2007 and December 2018. Seventeen patients were excluded, six of them did not have evaluable pre-treatment imaging and eleven patients eventually did not receive cisplatin-based chemoradiotherapy. Table 1 shows the characteristics of the included patients. Of the included patients, 235 patients (68.5%) were male and the median age at diagnosis was 59.07 years (IQR 53.41–63.70).
Known risk factors for HNC are smoking and alcohol use, which is also seen in this study. Majority of patients smoked (n = 282, 82.2%) and used alcohol (n = 283, 82.5%). In the selection of patients fit for cisplatin treatment, the medical oncologist takes into consideration patients’ comorbidities. This is represented by the minority of patients (n = 34, 9.9%) who had severe comorbidities, as evaluated by the ACE-27 comorbidity score, in this study. Most patients (n = 144, 42%) were symptomatic but completely ambulatory as indicated by the ECOG performance status of 1.
Cisplatin-based chemoradiotherapy is most frequently given in a primary treatment setting in patients with LA-HNC. As previously mentioned, adjuvant chemoradiotherapy is only advised when the tumor is irradically resected or in the presence of extracapsular lymph-nodal extension. In this study, the majority of patients were treated in a primary setting (n = 274, 79.9%) and had a tumor, node, metastasis (TNM) stage IV tumor according to the 7th edition TNM cancer staging criteria (n = 284, 82.8%).
Prior to initiation of chemoradiotherapy the mean biochemical values of the patients were as follows: mean hemoglobin of 8.5 mmol/L (SD 1.1), mean serum creatinine of 69.9 μmol/L (SD 15.6), mean serum albumin of 40.0 g/L (SD 4.9) and mean total protein of 71.2 g/L (SD 7.9).
Anthropometric measurements
Table 2 shows the anthropometric measurements of the included patients. Of the 343 included patients, 199 patients (58.0%) had low SMM at diagnosis. The median LSMI was 41.6 cm2/m2 (IQR 35.4–45.5). The median LBM was 44.8 kg (IQR 37.1–50.6). Majority of patients (n = 191, 55.7%) had ad normal weight as indicated by the body mass index (BMI) of 18.5–24.9 kg/m2. The median body surface area at diagnosis was 1.9 m2 (IQR 1.7–2.0).
Low skeletal muscle mass
Table 1 shows the differences in demographic, clinical and biochemical characteristics between patients with and without low SMM (LSMI ≤ 43.2 cm2/m2) at diagnosis. Demographical and clinical characteristics which were significantly more likely to be present in patients with low SMM were; being of female gender (n = 103, 95.4%; p < 0.01), older age at diagnosis (59.4 years; p < 0.01), smoking (n = 173, 61.3%; p = 0.01), an ECOG performance status of ≥ 2 (n = 24, 63.2%; p = 0.02) and being treated in an adjuvant chemoradiotherapy setting (n = 48, 69.6%, p = 0.04).
In comparison to patients without low SMM, patients with low SMM were more likely to have lower mean albumin levels (38.4 g/L vs 39.9 g/L; p < 0.05), lower mean hemoglobin levels (8.2 mmol/L vs 8.9 mmol/L; p < 0.01) and lower mean serum creatine levels (65.1 μmol/L vs 76.8 μmol/L; p < 0.01). Interestingly, patients with low SMM at diagnosis received significantly higher cumulative doses of cisplatin per kilogram of LBM compared to patients without low SMM (9.0 mg/kg LBM versus 7.4 mg/kg LBM, p < 0.0001).
Table 2 shows the differences in anthropometric measurements between patients with low SMM at diagnosis and patients without low SMM. All underweight patients (BMI < 18.5 kg/m2) (n = 30, 8.7%) had low SMM. Patients without low SMM were more likely to be overweight (65.5%; p < 0.01) and obese (73.7%; p < 0.01).
Cisplatin dose-limiting toxicity
Of the 343 included patients, 154 patients (44.9%) experienced cisplatin DLT. Figure 2 shows a boxplot of the amount of SMM expressed as LSMI (cm2/m2) in patients who have not experienced cisplatin DLT and patients who experienced cisplatin DLT. Table 3 shows the types of cisplatin DLT categorized into patients with low SMM and without low SMM. Of the 154 patients that experienced DLT, in 145 patients (94.2%) this was due to the failure to complete all (n = 3) cycles of cisplatin, in 6 patients (3.9%) this was due to a treatment delay of ≥ 4 days and in 3 patients this was due to an cisplatin de-escalation of ≥ 50% (1.9%). The causes of cisplatin DLT were ototoxicity (n = 64, 41.6%), nephrotoxicity (n = 41, 26.6%), malaise (n = 29, 18.8%), hematopoietic toxicity (n = 12, 7.8%), vascular toxicity (n = 6, 3.9%) and neurotoxicity (n = 1, 0.6%).
Patients with low SMM were more likely to experience cisplatin DLT (n = 102, 66.2%) compared to patients without low SMM (n = 52, 33.8%) (p < 0.01). When comparing the causes of cisplatin DLT with SMM status, patients with low SMM were, in particular, more likely to not complete all cycles (n = 3) of cisplatin (n = 95, 65.5%) compared to patients without low SMM (n = 50, 34.5%) (p = 0.02). Patients who experienced cisplatin DLT were shown to have received significantly higher cisplatin doses per kg of LBM.
Table 1 and Table 2 show the differences in demographic, clinical, biochemical and anthropometric characteristic between patients who experienced cisplatin DLT and patients who did not experience cisplatin DLT. The SMM (LSMI) was significantly lower in patients with cisplatin DLT compared to patients without cisplatin DLT (LSMI 39.7 cm2/m2 versus 42.4 cm2/m2; p < 0.01). Female patients were more likely to experience cisplatin DLT (n = 60, 55.6%; p < 0.01). No significant differences were seen in other demographic, clinical or biochemical characteristics. Interestingly, although cisplatin is currently dosed on body surface area, it was not significantly different between patients whom experienced cisplatin DLT (1.9 m2) and patients whom did not experience cisplatin DLT (1.9 m2) (p = 0.2). However, LBM was significantly different between these patients (p < 0.05). Patients whom experienced cisplatin DLT had significantly lower mean LBM (42.7 kg) compared to patients who did not experience cisplatin DLT (mean LBM 45.1 kg) (p = 0.01). Patients whom were underweight (n = 30, 8.7%) were also more likely to experience cisplatin DLT (n = 18, 60%; p = 0.03).
Predictors for cisplatin dose-limiting toxicity
Table 4 shows the univariate and multivariate logistic regression analysis of the predictors for cisplatin DLT. In univariate analysis, significant predictors for increased risk of cisplatin DLT were female gender (OR 1.88; 95% CI 1.18–2.97; p < 0.01), LBM (OR 0.97; 95% CI 0.95–0.99; p = 0.01) and low SMM at diagnosis (OR 1.20; 95% CI 1.20–2.89, p < 0.01). Patients’ body surface area was not predictive for cisplatin DLT (HR 0.7, 95% CI 0.4–1.4; p = 0.4). Subsequently, low SMM was included in the multivariate analysis with the clinically relevant variables; age at diagnosis and BMI. Female gender was not included in the multivariate analysis because 95.4% of patients with low SMM were female patients. The LBM was not included because LBM is calculated by use of SMA in the Mourtzakis formula, SMA is already represented in SMM. In multivariate analysis, low SMM at diagnosis (OR 1.75; 95% CI 1.06–2.90, p = 0.03) remained the only significant predictive factor for cisplatin DLT. The Hosmer and LemesHow test showed that the multivariate analysis model had a high goodness-of-fit (Chi-square 8.11, p = 0.42).
Discussion
In this large retrospective study, we evaluated the association between low SMM prior to treatment with cisplatin-based chemoradiotherapy and the occurrence of cisplatin DLTs. We found that patients with low SMM at diagnosis were at significant risk for experiencing cisplatin DLTs compared to patients without low SMM. Cisplatin DLTs lead to failure of the intended treatment plan in 44.9% of patients.
Our findings are in line with previous studies in patients with LA-HNC [8, 19]. Our previous study in a smaller cohort of LA-HNC patients treated with either cisplatin or carboplatin showed a threefold increase in DLT frequency in patients with low SMM [8]. An association between low SMM and DLT has also been found in patients with non-small cell lung cancer, breast cancer, colorectal cancer, esophagogastric cancer and pancreatic cancer [9,10,11, 20, 21]. The scale of increased risk for DLTs found in these studies varies, mainly depending on the type of cytotoxic agent used and the cut-off points used to define low SMM.
Several hypotheses have been proposed in the literature to explain the underlying mechanism of this important finding in several types of cancers [13, 22]. The most accepted hypothesis is based on the influence of low SMM on the volume of distribution of anti-cancer drugs and assumes that dosing of anti-cancer drugs on body surface area is insufficient to capture body composition differences. Dosing cytotoxic agents on body surface area was initially derived from observations that basal metabolic rates non-linearly differed between species (humans, animals) according to weight [6]. These observations also showed that the maximum tolerated dose expressed as mg/m2 was similar in different species [6]. Therefore, in the 1950’s, body surface area (m2) calculated with patient’s body weight and body height was used as an estimate for safe starting doses in phase 1 human trials based on preclinical animal toxicology studies [6]. However, the use of body surface area for predicting a safe starting dose was extended as a dosing tool for cytotoxic agents. Prado et al. showed that LBM has a poor association with body surface area (r2 = 0.37) in patients with solid tumors of the respiratory or gastro-intestinal tract. They estimated that the individual variation in LBM could account for up to a threefold variation in volume distribution for anticancer drugs dosed per unit body surface area [23].
Currently, the best tool used to predict who will benefit from chemotherapy is the performance status of the patient, which can be measured by the Eastern Cooperative Oncology group (ECOG) or the Karnofsky performance status. Besides the performance status, patients’ comorbidities such as renal conditions and otologic conditions are taken into consideration as objective measures to classify a cisplatin-fit patient. However, the assessment of performance status by the clinician may be a subjective measure. In our study, also partly because unfit patients do not receive cisplatin, the performance status was, as expected, not an independent predictor for DLT. Besides performance status, BMI is mostly used as a surrogate measure of patients’ physical fitness or nutritional status in clinical oncology practice. We found that low BMI at diagnosis was not associated with an increased risk of DLTs. BMI is not an appropriate measurement tool to identify patient at risk for DLTs and may unjustly reassure oncologists about patients’ nutritional status and risk for experiencing adverse treatment effects. Ideally in the future, the body composition rather than the body weight should be taken into account during the diagnostic, treatment and surveillance stages of phases in oncology. A need for a more objective and integrated measurement tool, such as SMM assessment, is needed. This enables an individualized patient approach, as wide variations in body composition, especially SMM, are reported in many populations [24]. SMM can be determined on routinely performed diagnostic imaging and therefore may be useful in clinical practice to identify patients at risk for DLTs without additional patient burden.
Our study had some limitations. First, due to the retrospective design of this study no information was available on nutritional status and physical exercise, which may influence the relationship between SMM and DLT. Second, due to the observative nature of this study no causal relationship between cisplatin pharmacokinetics and body surface area or SMM could be drawn from this study and further prospective studies are needed to elucidate this relationship. Thirdly, due to the discrepancy of males and females in this study, 235 males and 108 females, we did not calculate gender-specific cut-off values of low SMM. SMM may, however, differ between males and females. Further studies should be performed to determine valid gender-specific cut-off values for low SMM in HNC patients.
Early screening to identify patients with occult low SMM combined with multimodal interventions may offer an improvement in treatment tolerance. To improve treatment tolerance to chemoradiotherapy in patients with LA-HNC two possible solutions are worth investigating: I. A new concept of cisplatin drug dosing schedules per kilogram of LBM using C3 muscle area measured on CT or MRI and II. SMM improvement by a multimodal approach including physical exercise (aerobic and resistance training), nutritional supplements (high protein) and pharmacological agents (anti-inflammatory, detoxifying agents).
In conclusion, the current method of dosing cisplatin in patients with LA-HNC leads to the observed high frequency of DLT which may impair tumor treatment and definitely impairs quality of life. Low SMM at diagnosis is highly predictive for DLT. Cisplatin dosing based on taking SMM into account may be a promising new concept in HNC to improve treatment tolerance.
Abbreviations
- LA-HNC:
-
Locally advanced head and neck cancer
- SMA:
-
Skeletal muscle area
- SMM:
-
Skeletal muscle mass
- LSMI:
-
Lumbar skeletal muscle index
- C3:
-
Third cervical vertebra
- L3:
-
Third lumbar vertebra
- HU:
-
Hounsfield unit
- LBM:
-
Lean body mass
- DLT:
-
Dose-limiting toxicity
References
Ferlay J, Colombet M, Soerjomataram I et al (2018) Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 103:356–387. https://doi.org/10.1016/j.ejca.2018.07.005
Pressoir M, Desné S, Berchery D et al (2010) Prevalence, risk factors and clinical implications of malnutrition in french comprehensive cancer centres. Br J Cancer 102(6):966–971. https://doi.org/10.1038/sj.bjc.6605578
Elliott JA, Doyle SL, Murphy CF et al (2017) Sarcopenia: prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann Surg 266(5):822–830. https://doi.org/10.1097/SLA.0000000000002398
Chargi N, Bril SI, Swartz JE, Wegner I, Willems SW, de Bree R (2020) Skeletal muscle mass is an imaging biomarker for decreased survival in patients with oropharyngeal squamous cell carcinoma. Oral Oncol. https://doi.org/10.1016/j.oraloncology.2019.104519
Ansari E, Chargi N, van Gemert JTM et al (2020) Low skeletal muscle mass is a strong predictive factor for surgical complications and a prognostic factor in oral cancer patients undergoing mandibular reconstruction with a free fibula flap. Oral Oncol 101:104530. https://doi.org/10.1016/j.oraloncology.2019.104530
Sawyer M, Ratain MJ (2001) Body surface area as a determinant of pharmacokinetics and drug dosing. Invest New Drugs 19(2):171–177. https://doi.org/10.1023/A:1010639201787
Astolfi L, Ghiselli S, Guaran V et al (2013) Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: a retrospective evaluation. Oncol Rep 29(4):1285–1292. https://doi.org/10.3892/or.2013.2279
Wendrich AW, Swartz JE, Bril SI et al (2017) Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol 71:26–33. https://doi.org/10.1016/j.oraloncology.2017.05.012
Ali R, Baracos VE, Sawyer MB et al (2016) Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 5(4):607–616. https://doi.org/10.1002/cam4.621
Sjøblom B, Grønberg BH, Benth JŠ et al (2015) Low muscle mass is associated with chemotherapy-induced haematological toxicity in advanced non-small cell lung cancer. Lung Cancer 90(1):85–91. https://doi.org/10.1016/j.lungcan.2015.07.001
Kurk S, Peeters P, Stellato R et al (2019) Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle 10(4):803–813. https://doi.org/10.1002/jcsm.12436
Hopkins JJ, Sawyer MB (2017) A review of body composition and pharmacokinetics in oncology. Expert Rev Clin Pharmacol 10(9):947–956. https://doi.org/10.1080/17512433.2017.1347503
Hilmi M, Jouinot A, Burns R et al (2019) Body composition and sarcopenia: the next-generation of personalized oncology and pharmacology? Pharmacol Ther 196:135–159. https://doi.org/10.1016/j.pharmthera.2018.12.003
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85(1):115-122. https://doi.org/10.1152/jappl.1998.85.1.115
Chargi N, Ansari E, Huiskamp LFJ, Bol G, de Bree R (2019) Agreement between skeletal muscle mass measurements using computed tomography imaging and magnetic resonance imaging in head and neck cancer patients. Oral Oncol 99:104341. https://doi.org/10.1016/j.oraloncology.2019.06.022
Swartz JE, Pothen AJ, Wegner I et al (2016) Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol 62:28–33. https://doi.org/10.1016/j.oraloncology.2016.09.006
Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33(5):997–1006. https://doi.org/10.1139/H08-075
Baumgartner RN, Koehler KM, Gallagher D et al (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147(8):755–763. https://doi.org/10.1093/oxfordjournals.aje.a009520
Sealy MJ, Dechaphunkul T, Van Der Schans CP et al (2019) Low muscle mass is associated with early termination of chemotherapy related to toxicity in patients with head and neck cancer. Clin Nutr 39(2):501–509. https://doi.org/10.1016/j.clnu.2019.02.029
Dijksterhuis WPM, Pruijt MJ, van der Woude SO et al (2019) Association between body composition, survival, and toxicity in advanced esophagogastric cancer patients receiving palliative chemotherapy. J Cachexia Sarcopenia Muscle 10(1):199–206. https://doi.org/10.1002/jcsm.12371
Basile D, Corvaja C, Caccialanza R, Aprile G (2019) Sarcopenia: Looking to muscle mass to better manage pancreatic cancer patients. Curr Opin Support Palliat Care 13(4):279–285. https://doi.org/10.1097/SPC.0000000000000455
Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E (2018) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 37(4):1101–1113. https://doi.org/10.1016/j.clnu.2017.07.010
Prado CM, Lieffers JR, McCargar LJ et al (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9(7):629–635. https://doi.org/10.1016/S1470-2045(08)70153-0
Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS (2001) Sarcopenia. J Lab Clin Med 137(4):231–243. https://doi.org/10.1067/mlc.2001.113504
Funding
No financial support was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chargi, N., Bashiri, F., Wendrich, A.W. et al. Image-based analysis of skeletal muscle mass predicts cisplatin dose-limiting toxicity in patients with locally advanced head and neck cancer. Eur Arch Otorhinolaryngol 279, 3685–3694 (2022). https://doi.org/10.1007/s00405-021-07229-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-07229-y