Abstract
Objective
The objective of this study was to evaluate the clinical outcomes of conventional two-dimensional (2D) endoscope with a novel computer-based three-dimensional (3D) imaging system for otologic surgical procedures.
Methods
A conventional 2D monocular endoscope with a novel computer-based 3D imaging system was applied to 18 otologic surgical procedures, including chronic otitis media (COM), cholesteatoma, otosclerosis, external canal osteoma and cochlear implant. Operation duration and complications of COM and attic cholesteatoma were recorded to compare 2D and 3D endoscopic ear surgery. Questionnaires were completed by 35 observers participating in the procedures and were used to evaluate clinical and potential side effects.
Results
The surgical procedures were performed smoothly for all patients. No patient required switching to conventional 2D endoscopic surgery. No significant differences were apparent in operation duration using the 3D imaging system for chronic otitis media and attic cholesteatoma compared with conventional 2D endoscopic ear surgery. Thirty-five observers completed the questionnaires. Most of them agreed that this 3D imaging system enabled them to perceive stereoscopic vision (94%), provide superior depth perception (85%). Furthermore, 97.1% reported no visual fatigue or discomfort when observing the 3D images.
Conclusion
Our study demonstrated that the computer-based 3D imaging system enables the application of 3D vision technology to otologic surgery. The system has no obvious side effects, such as visual fatigue or time delay. It not only facilitates performing the related surgical procedures but also helps in teaching and learning endoscopic ear surgeries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, endoscopic ear surgeries have been more popular and widely used [1,2,3,4]. The endoscopic surgery overcomes the limitations of conventional microscopic ear surgery and offers greater accessibility to a narrow external auditory meatus or a prominent anterior bony overhang of the ear canal. Moreover, an endoscope offers improved visualization of the middle ear because the light source is located at the distal tip of the scope [5,6,7,8,9]. Although endoscopic ear surgery is widely applied, it does not provide the stereoscopic vision offered by microscopic ear surgery. Endoscopic surgeons learn to overcome this limitation through a series of visual cues that include the movement of the endoscope forward and backward in the operative field and the interaction of the surgical instruments with anatomic structures. Despite this adjustment, the depth perception provided by endoscopic ear surgery is not as precise as that offered by microscopic ear surgery. Therefore, a 3-dimensional (3D) imaging system was developed to provide the depth perception and regain stereoscopic vision.
A 3D imaging system with a binocular technique has been introduced for laparoscopic surgery [10], sinus surgery [11, 12], and skull base surgery [13]. However, it is not considered appropriate for endoscopic ear surgery because of the precise maneuvers at close range and the thin endoscope required for the procedure. This is because of the intrinsic limitation of the binocular technique, a large binocular parallax, which leads to double vision when applied at a close range. Up to now, there was only one clinical pilot study about 3D binocular 4 mm endoscope applied in ear surgeries for 8 patients, but there was no comparison of the outcomes between 2D and 3D technologies [14]. In 2013, a monocular 3D endoscope, named VS3 system (Visionsense, Petach Tikva, Israel), was developed and applied to sinus surgeries [15, 16]. This system uses the “insect eye” technology that incorporates a microscopic array of lenses in front of a single video chip at the end of the endoscope, acting as separate visual receptors, in turn generates 3D vision. The VS3 system is also 4 mm in diameter, making it theoretically applicable to endoscopic ear surgery and a suitable alternative to the aforementioned system. However, no evidence of clinical ear surgeries practice could be assessed so far. In 2015, a novel imaging system with a computer-based processor to generate 3D images was first introduced for transurethral endoscopic surgery [17]. This imaging processor converts the single signal input from a 2D endoscope to a pair of images and mimics binocular vision to create a 3D visualization. We hypothesize that this system is another option for endoscopic ear surgery because the processor can generate 3D images in real time according to the input of the algorithm from a conventional two-dimensional (2D) endoscopic image. The processor converts the conventional 2D endoscopic image into a pair of images, as received from two viewpoints, to imitate the parallax of the eyes [17]. The major breakthrough of the technique was that the imaging system could be applied with any conventional monocular 2D endoscope. Therefore, a smaller diameter (less than 4 mm) 2D endoscope equipped with a novel 3D imaging system can enable ear surgeons to operate with 3D vision.
This is the first study to apply the computer-based 3D imaging system to endoscopic ear surgeries. Herein, we discuss the preliminary clinical outcomes and potential side effects exhibited in 18 patients. We also compared the surgical outcomes and the operation time of chronic otitis media and cholesteatoma surgeries based on 2D and 3D technologies.
Materials and methods
Participants
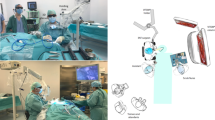
This study is a continuous case series. Computer-based processing 3D imaging system was applied to six patients with chronic otitis media (COM), two with bilateral profound hearing loss, one with otosclerosis, one with congenital cholesteatoma, two with external auditory canal osteoma, and six with attic cholesteatoma from December 2015 to December 2017. The 3D system used was provided by Unique Medical International Co. Ltd., at available days. None of the participants received any financial support. The demographic characteristics of patients were shown in (Table 1). All procedures for these patients were performed by the same senior surgeon. A total of 35 attendees observed 18 surgical procedures performed by the medical team. Throughout the entire procedure, the surgeon and all observers wore stereoscopic glasses for 3D vision (Fig. 1).
Interventions
Endoscopic ear surgery was performed using a computer-based 3D imaging system, Novel HD-3D-A (Shinko Optical, Japan). The 3D images were displayed on monitor (LMD-2451 MT 3D; Sony, Japan) as shown in left side of Fig. 1. The 2D images were also acquired using a 3-chip video camera and were displayed simultaneously on another LCD monitor (Karl Storz, Germany) (in the center of Fig. 1). The 2D and 3D images were simultaneously displayed on separate monitors during the entire operation. Therefore, observers are able to take off their goggles during the operative procedure to compare the 2D and 3D images of the operative field and to perceive any change in brightness, sharpness and colors, and time delay. The Hopkins® Straight Forward Telescope 0° 2D endoscope (diameter, 3 mm; length, 14 cm; Karl Storz) was used for most procedures; the Hopkins® Forward Oblique Telescope 45° was applied for assistance, and Hopkins® Straight Forward Telescope 0° 2D endoscope (diameter, 1.9 mm; length, 10 cm; Karl Storz) was used for posterior tympanotomy in cochlear implant (CI) surgery.
Outcome assessment and statistical analyses
The operation times for patients with COM and attic cholesteatoma using the 2D/3D imaging system were recorded, and surgical outcomes were compared. Operation time was calculated beginning from skin incision and ending when a cotton ball was packed into the external ear canal. By matching with age and the severity of disease (including the perforation size of COM, the size of cholesteatoma and the pure tone audiometry threshold), we selected 12 patients with simple COM and 12 patients with attic cholesteatoma to undergo a similar surgical procedure using a 2D endoscope in the same period and compared the operation times and surgical complications. Statistical analysis was performed using Statistical Package for Social Science version 16 for Windows (SPSS, Inc, an IBM Company, Chicago). The study results are expressed as mean and standard deviation. We compared the study data using the Mann–Whitney U test. Differences were considered significant when p < 0.05.
Potential disadvantages of the 3D imaging system are time delay and eye fatigue. Time delay during operations may negatively affect the safety and efficiency of the surgery. On the other hand, stereoscopic vision can help to process spatial perception quickly, and enable the performance of critical procedures during an operation in seconds. Using stereoscopic vision, especially in ossiculoplasty, we can see exactly where spotting objects are located in relation to their surroundings with precise depth perception. Additionally, if a surgeon experiences eye fatigue easily from staring at the monitor for a period of time, it may adversely affect the performance of the surgery.
The advantages of the 3D imaging system were assessed through questionnaires distributed to all the observers who were requested to evaluate the clinical utility and potential side effects of the system. The observers were asked to comment on the flowing items: (1) Does this 3D imaging system let you perceive stereoscopic vision? (“agree/disagree”); (2) Does this 3D imaging system provide superior depth perception compared to a conventional 2D endoscope? (“agree/fair/disagree”); and (3) Do you feel visual fatigue or discomfort when observing 3D imaging? (“yes/no”). The questionnaires were administered immediately following completion of the procedure. Some observers attended more than one surgical procedure, but they did not repeat the questionnaire. A total of 35 responses were returned and analyzed. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, and the experiment was conducted in accordance with the Declaration of Helsinki.
Results
The surgical procedures were performed smoothly for all 18 patients. The surgeon wore the 3D goggles during the whole surgical procedure and he could judge with his experiences if this system provides adequate stereoscopic vision and depth perception or not. For all 18 patients, no surgical steps required switching to conventional 2D endoscopic surgery to complete the surgery. No mortality, perioperative complications, or other notable postoperative complications, such as infection, massive bleeding, hematoma, post-operative hearing loss occurred in this study. Operation time was 48.55 ± 20.46 and 45.6 ± 16.9 min for 2D and 3D tympanoplasty type I, respectively. There were no significant differences in the operation time between 2D and 3D type I tympanoplasty (p = 0.457, Mann–Whitney U test) as displayed in Table 2. For attic cholesteatoma, no significant differences were evident between the 3D and conventional 2D endoscopic ear surgery methods (p = 0.285, Mann–Whitney U test); however, the 3D group (105.6 ± 20.6 min) exhibited a trend of shorter operating time than 2D group (117.7 ± 27.1 min). Figure 2 illustrates examples of 2D and 3D images of posterior tympanotomy during the CI surgery. We performed the cochlear implant surgery with combined microscopic and endoscopic approaches. After mastoidectomy and posterior tympanotomy, the endoscope (1.9 mm) was used to accurately locate the round window with close view and assist electrode insertion with extend round window approach. Under 2D image system, the operator would have to move the endoscope in and out slightly to create 3D perception. But with 3D system, the operator could see 3D image even when the endoscope is static and the measurement of the distance between the related structures is more precise. The 3D imaging system achieved superior depth perception and a clearer depiction of surgical anatomy, especially the distance between ossicles, sinus tympani, facial recess, and their surroundings. The surgeon did not perceive a time delay at any time during the surgical procedures. This computer processing system transfers the images of 2D endoscopic system into 3D images. Therefore, the surgeon will not be disturbed by double vision which did not depend on target distances during the whole procedure.
Questionnaires were completed by 35 observers right after completing the operation. Among them, 33 (94.3%) agreed that this 3D imaging system enabled them to perceive stereoscopic vision, whereas 2 (5.7%) disagreed with this description, as shown in Fig. 3. Moreover, 30 observers (85.7%) agreed that this system provided superior depth perception than did a conventional 2D endoscope; 4 (11.4%) considered that the system only provided fair depth perception, and 1 (2.9%) disagreed with this description. The same observer (2.9%) experienced visual fatigue or discomfort when observing the 3D images, but the remaining 34 (97.1%) reported no visual fatigue or discomfort.
Discussion
This is the first practical study in which a surgeon performed endoscopic ear surgery using 3-D computer-based processing system. This 3-D imaging system can also be combined with any conventional 2D endoscope to perform various procedures such as transcanal ear surgery. We compared the clinical outcomes and complications between patients receiving the 2D and 3D endoscopic ear surgeries, and our results indicated that no significant differences were evident with respect to the operating time of simple COM and attic cholesteatoma procedures. However, we discerned a trend in which 3D atticotomy and ossiculoplasty procedures were performed in a shorter operating time. This may imply that the 3D imaging system provided superior depth perception and a clearer depiction of surgical anatomy, which thereby shortened the operating time for more complicated surgical procedures.
The surgeon and 94.3% of the observers agreed that this 3D imaging system enabled them to perceive stereoscopic vision. The 2D and 3D images were displayed on separate monitors simultaneously, and the observers were able to analyze 2D and 3D images easily; thus, they could determine whether the 3D image provided stereoscopic vision and enhanced depth perception. Furthermore, the surgeon and 85.7% of the observers opined that this imaging system provided superior depth perception, and the clear depiction of anatomical structures could aid the performance of procedures. The estimated distances between the related structures are more precise with stereoscopic perception in 3D image system. This advantage would be optimal when operating in small spaces and doing exquisite surgical steps, such as inserting the electrode into the cochlea or cramping the piston ring. The precise estimation of the distance is important and helpful for inexperienced surgeons.
The average diameter of the external ear canal is only 7 mm on average, and thus the proper caliber of endoscope may be restricted [18]. The computer-based 3D imaging system could be applied with any conventional monocular 2D endoscope, thereby obviating any concerns about the diameters of endoscopes. We also paired this system with a 3 mm conventional 2D endoscope to perform various procedures on patients from 2 to 76 years old; in the youngest patient, a 2-year-old child, congenital cholesteatoma was successfully removed without widening the ear canal.
We also compare the cost of the different 3D imaging system available in the market now. The cost of the equipment of a 3D imaging system with a binocular technique and a monocular 3D endoscope with the “insect eye” technology (VS3 system) are at least twice the price of the computer-based processing system. Besides, this computer-processing 3D system can be applied to any caliber of previous 2D endoscope (4 mm, 3 mm, or even 1.9 mm) without necessity of new endoscope. Therefore, the total cost of the equipment is much cheaper than another two 3D systems.
There were some potential side effects. Firstly, Ogino-Nishimura et al. had reported that excessive blood loss would lead to decreased visual field brightness in endoscopic surgeries [12]. This phenomenon was also noted during our procedures but was inconsequential in our operations. The quantity of blood loss is limited in ear surgeries, and an experienced operator could adapt to the change in brightness rapidly.
Secondly, Kumar et al. reported that the long computation processing time could cause image time delay during fast endoscopic movement [10]. Because the ear canal is rigid and bony and a constricted space, endoscopes should be moved carefully and slowly during an operation; thus, the speed of movement is not a major concern and the time delay problem can be avoided. We did not encounter it in our practice.
Thirdly, regarding visual fatigue, Yamauchi et al. assessed the incidence of fatigue in surgeons using a stereoscopic endoscope and reported no difference between 3D and 2D stereoscopic endoscopes [19]. Both of them had a similar operative time of approximately 1 h. In our study, only one observer (1/35, 2.9%) reported fatigue, whereas the other observers did not. Otologic surgeries usually last for several hours; therefore, it is important that surgeons do not experience visual fatigue, which may affect the performance of surgery adversely.
Although, the observers did not conduct the procedures most of them agreed that this 3D imaging system enabled them to perceive stereoscopic vision and has superior depth perception when compared with a conventional 2D endoscope. Therefore, consensus was achieved regarding the 3D imaging system offering a clearer understanding of the anatomical structures, which can facilitate their learning.
This study had several limitations. First, the number of subjects in this study is limited. Although the data were collected for 18 patients and subjected to statistical analysis to evaluate the clinical outcomes of this 3D system, the effective systematic analysis was only performed to compare two types of surgeries. Because the 18 patients underwent different type of ear surgery, including tympanoplasty, mastoidectomy, ossiculoplasty, cochlear implant, the numbers of subjects in each type of ear surgery are very limited, and thus the data are statistically insufficient to evaluate the system’s effectiveness for each surgical application. Therefore, a prospective randomized trial should be conducted to validate the present findings in the future. Second, endoscopic technology is rapidly developing, which may lead to the invention of thinner scopes and more advanced methods of image capture and transmission in the future. This would affect the clinical application of this 3D imaging system. Despite these limitations, this is the first study of the 3D image system applied to endoscopic ear surgeries. This 3D imaging system is also suitable for use in pediatric patients if a conventional 2D endoscope of a small caliber is employed. We used this 3D system to perform several types of ear surgeries, and proved that the 3D system is feasible for application in various endoscopic ear surgeries. We expect the present findings to serve as a crucial reference to guide the design of future trials aimed at investigating the feasibility of 3D video-assisted endoscopy in otologic surgeries.
Conclusion
This is the first study to perform endoscopic ear surgery using 3D computer-based processing system. Our preliminary results indicate that the proposed computer-based 3D imaging system overcomes the inferior depth perception of the 2D endoscope, which is its major limitation. Furthermore, the system has no obvious side effects such as visual fatigue or time delay. This 3D processing technology not only facilitates performance of the surgical procedures but also aids in the teaching and learning of endoscopic surgery. Additional large-scale studies are suggested to further assess the effectiveness of this 3D imaging system for endoscopic ear surgery.
References
Marchioni D, Alicandri-Ciufelli M, Gioacchini FM, Bonali M, Presutti L (2013) Transcanal endoscopic treatment of benign middle ear neoplasms. Eur Arch Otorhinolaryngol 270(12):2997–3004
Migirov L, Wolf M (2013) Endoscopic transcanal stapedotomy: how I do it. Eur Arch of Otorhinolaryngol 270(4):1547–1549
Tarabichi M, Ayache S, Nogueira JF, Al Qahtani M, Pothier D (2013) Endoscopic management of chronic otitis media and tympanoplasty. Otolaryngol Clin North Am 46(2):155–163
Kiringgoda R, Kozin ED, Lee DJ (2016) Outcomes in endoscopic ear surgery. Otolaryngol Clin North Am 49:1271–1290
Kozin ED, Lee DJ (2015) Transcanal endoscopic ear surgery: a new era of otologic surgery? Hear J 68:8–12
Kozin ED, Gulati S, Kaplan AB et al (2014) Systematic review of outcomes following observational and operative endoscopic middle ear surgery. Laryngoscope 125:1205–1214
Tarabichi M (2010) Endoscopic transcanal middle ear surgery. Indian J Otolaryngol Head Neck Surg 62:6–24
Marchioni D, Soloperto D, Rubini A et al (2015) Endoscopic exclusive transcanal approach to the tympanic cavity cholesteatoma in pediatric patients: Our experience. Int J Pediatr Otorhinolaryngol 79:316–322
Presutti L, Gioacchini FM, Alicandri-Ciufelli M, Villari D, Marchioni D (2014) Results of endoscopic middle ear surgery for cholesteatoma treatment: a systematic review. Acta Otorhinolaryngol Ital 34:153–157
Kumar A, Wang Y, Wu C, Liu K, Wu H (2014) Stereoscopic visualization of laparoscope image using depth information from 3D model. Comput Methods Progr Biomed 113:862–868
Albrecht T, Baumann I, Plinkert P, Simon C, Sertel. S (2016) Three-dimensional endoscopic visualization in functional endoscopic sinus surgery. Eur Arch Otorhinolaryngol 273:3753–3758
Ogino-Nishimura E, Nakagawa T, Sakamoto T, Ito J (2015) Efficacy of three-dimensional endoscopy in endonasal surgery. Auris Nasus Larynx 42:203–207
Chan JYW, Wei WI (2018) Three-dimensional endoscopy for endoscopic salvage nasopharyngectomy: preliminary report of experience. Laryngoscope 128(6):1386–1391
Bernardeschi D, Lahlou G, De Seta D, Russo FY, Mosnier I, Sterkers O (2018) 3D endoscopic ear surgery: a clinical pilot study. Eur Arch Otorhinolaryngol 275(2):379–384
Brown SM, Tabaee A, Singh A, Schwartz TH, Anand VK (2008) Three-dimensional endoscopic sinus surgery: feasibility and technical aspects. Otolaryngol Head Neck Surg 138:400–402
Szold A (2005) Seeing is believing: visualization systems in endoscopic surgery. Surg Endosc 19:730–733
Yoshida S, Kihara K, Fukuyo T, Ishioka J, Saito K, Fujii Y (2015) Novel three-dimensional image system for transurethral surgery. Int J Urol 22:714–715
Clark WW, Ohlemiller KK (2008) Structural and functional anatomy of the outer and middle ear. Anatomy and physiology of hearing for audiologists. Thomson Delmar, Clifton Park, pp 93–108
Yamauchi Y, Shinohara K (2005) Evaluation of fatigue attributed to binocular stereopsis on surgical tasks with a stereoscopic endoscope. J Jpn Soc Comput Aided Surg 7:115–125
Funding
This work was supported, in part, by Chang Gung Memorial Hospital under Grant Nos. CMRPG3C1711-3, CMRPG3E0981 and by the National Science Council of the Republic of China (Taiwan) under Grant No. 102-2221-E-182A-004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Rights and permissions
About this article
Cite this article
Chen, CK., Hsieh, LC. & Hsu, TH. Novel three-dimensional image system for endoscopic ear surgery. Eur Arch Otorhinolaryngol 275, 2933–2939 (2018). https://doi.org/10.1007/s00405-018-5153-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-5153-7