Abstract
Purpose
Microbial biofilms have been implicated in the pathogenesis of chronic rhinosinusitis with nasal polyposis (CRSwNP). The aim of our study was to evaluate in vitro effects of amoxicillin–clavulanic acid and levofloxacin on biofilm formation by bacterial species isolated from sinus tissue in patients with CRSwNP.
Methods
The sinus mucosal specimens were harvested from the upper parts and roof of ethmoid cavity of 48 patients with CRSwNP. Each sample was washed thoroughly in three separate beakers of sterile saline to remove any planktonic bacteria and further subjected to microbiology analysis. The biofilm-forming capacity of isolated strains was detected by microtiter-plate method and the effects of subinhibitory (1/2× to 1/16× MIC) and suprainhibitory concentrations (4, 8, 16, 32, and 64 µg/ml) of amoxicillin–clavulanic acid and levofloxacin on biofilm production were investigated.
Results
Bacterial strains were isolated in 42 (87.5%) patients: one microorganism in 80.9% and two microorganisms in 19.1% of patients. The most prevalent bacteria in CRSwNP biofilms were Staphylococcus epidermidis (34%) and S. aureus (28%) followed by S. haemolyticus (12%), Pseudomonas aeruginosa (8%), Moraxella catarrhalis (6%), Streptococcus pneumoniae (6%), and other staphylococci (6%). Subinhibitory concentrations of amoxicillin–clavulanic acid and levofloxacin significantly reduced biofilm formation (p < 0.01 and p < 0.05, respectively), with better efficacy of amoxicillin–clavulanic acid (1/2–1/8× MIC) on staphylococci and levofloxacin (1/2– 1/4× MIC) on M. catarrhalis and P. aeruginosa biofilm formation. Suprainhibitory concentrations of both tested antibiotics (4–64 µg/ml) significantly eradicated mature biofilms of staphylococci (p < 0.01). The effect of levofloxacin on eradication of staphylococcal biofilms was more noticeable, compared to the effect of amoxicillin–clavulanic acid (p < 0.01). Suprainhibitory concentrations of both tested antibiotics had no effect on eradication of previously formed M. catarrhalis and P. aeruginosa biofilms (p > 0.05).
Conclusions
The amoxicillin–clavulanic acid and levofloxacin are shown to be potent antibiofilm agents in patients with CRSwNP. The effects of tested compounds depend on bacterial species and the volume of formed biofilm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is a clinical syndrome with 4.5 to 12% prevalence in North American and European countries, causing significant health and socioeconomic burden [1]. The disease has multifactorial etiology and is characterized by persistent symptomatic inflammation of the nasal cavities and paranasal sinuses mucosa, often accompanied by infection. CRS is divided into two phenotypes based on nasal endoscopy: CRS with nasal polyposis (CRSwNP) and CRS without nasal polyps (CRSsNP). The role of biofilms in both CRSsNP and CRSwNP pathogenesis, either as a trigger or a persistent stimulus for inflammation, has been previously described [2,3,4,5,6].
Biofilm is a multicellular community embedded in extracellular polymeric substance (EPS) attached to the biotic or abiotic surfaces. During the process of biofilm formation, starting from the attachment of planktonic bacteria, through proliferation, maturation and finally detachment of micro-parts of biofilms, bacteria in biofilms undergo different phases of gene expression and different metabolic states relative to their planktonic counterparts. This specific cytoarchitecture of biofilm contributes to its resistance to immunity as well as to the action of antimicrobial agents. Physical barrier of the EPS that diminishes diffusion of antibiotics, and dormant state of bacteria in the deeper layers of biofilm are the major causes of biofilm-insensitivity to antimicrobials [7]. This phenomenon is also enhanced by the extensive interchange of genetic material responsible for various mechanism of bacterial resistance [8, 9]. Biofilms in the sinonasal cavities have 10- to 1000-fold decreased susceptibility to antimicrobial agents compared to the sensitivity of planktonic bacteria of the same species [2].
Biofilm-associated infections in CRS patients are caused by various bacterial species, with a common feature of antibiotic resistance or multiresistance, due to selective pressure from frequent antibiotic therapy. Such diverse microbiota, combined with uncertain medical outcome of empiric antibiotic therapy represents a great challenge in treatment of these infections. Nowadays, medical treatment of CRS patients consists of a combination of nasal saline and topical drugs with anti-inflammatory effect, such as corticosteroids, with or without antibiotics or surgery. Despite the fact that no antibiotic has US Food and Drug Administration approval for use in CRS [10], amoxicillin–clavulanic acid and second- or third-generation cephalosporins are considered the first-line antibiotic choice for CRS exacerbations [11, 12]. The respiratory quinolones are helpful second-line agents for refractory cases, followed by trimethoprim–sulfamethoxazole, doxycycline, telithromycin, and macrolides [12]. Macrolide antibiotics, specifically 14-member ring macrolides (erythromycin, clarithromycin, and roxithromycin), deserve special mention in regards to chronic rhinosinusitis, due to their anti-inflammatory effects [13].Therefore, macrolides can be considered as a therapy for patients with recalcitrant disease despite surgery and/or maximal medical management. Nevertheless, due to the emergence of antibiotic resistance and increased failure rate of empiric treatment, current guidelines recommend culture-directed therapy [10, 12, 14].
Although topical or systemic antibiotic therapy is often prescribed to CRS patients, there is a serious lack of evidence regarding their efficacy [10]. Moreover, the role of antibiotics for treatment of CRS biofilms has not been sufficiently investigated, with only a few studies investigating antibiofilm activity of macrolides in vivo [15] or topical antibiotics such as moxifloxacin [16], ciprofloxacin, vancomycin, and mupirocin in vitro [17]. Therefore, the aim of the study was to evaluate in vitro effects of oral antibiotics amoxicillin–clavulanic acid and levofloxacin on biofilms of bacterial species isolated from sinus tissue in patients with CRSwNP.
Materials and methods
Study design and population
This study was undertaken during the period between May 2014 and May 2015 at the Clinic of Otorhinolaryngology and Maxillofacial Surgery, Clinical Centre of Serbia, Faculty of Medicine, referral centre for otorhinolaryngology diseases in Serbia. The investigation was approved by the Ethical Committee of Faculty of Medicine, University of Belgrade (No.29/IV-14).
Forty eight consecutive patients, who met the definition of CRSwNP as defined by the EPOS 2012 guidelines and undergoing endoscopic sinus surgery (ESS) [18], were included in the investigation. Patients were excluded if less than 18 years of age, or had decreased ciliary function and antibiotic or corticosteroids used in the 3 weeks preceding surgery. All included patients provided informed consent before enrollment.
Tissue collection, preparation, and homogenisation
During the operation, sinus mucosal specimens were harvested from the upper parts and roof of ethmoid cavity of individual patient. One portion of the specimens was used for microbiology investigation and remaining specimens were fixed in 4% formalin for hematoxylin-eosin staining. The specimens obtained from each patient were processed and analyzed in microbiology laboratory within 2 h of collection in accordance with standard microbiology operative procedures. Each sample was washed thoroughly in three separate beakers of sterile saline to remove any planktonic bacteria. Samples were homogenized in sterile saline with a sterile Teflon homogeniser. The homogenate was inoculated into Brain Heart Infusion broth (bioMérieux, France) and incubated at 37 °C for 24 h. Blood agar and MacConkey agar (bioMérieux) were streaked with the 24-h cultures and incubated under the same conditions.
Histopathological examination was performed in accordance with previously described protocol [19]. Biofilm appears as clusters of small basophilic bacteria and host cells entrapped in a layer of extracellular polymeric substance on the surface epithelium.
Bacterial identification and antimicrobial susceptibility testing
Identification of the bacterial strains was performed by MALDI-TOF Vitek MS System (bioMérieux). Antimicrobial susceptibility was determined by Vitek 2 automated system (bioMérieux) in accordance with the European Committee on Antimicrobial Susceptibility Testing recommendation (http://www.eucast.org). In addition, antimicrobial activity of amoxicillin–clavulanic acid and levofloxacin was determined by standard broth microdilution test. Antibiotics were sequentially diluted in fresh Mueller-Hinton broth (bioMérieux) with the addition of 0.05% triphenyl tetrazolium chloride (Sigma-Aldrich, Chemical Company Inc, USA) as a growth indicator, and inoculated in triplicate with 5 × 105 CFU/ml of bacteria. Minimum inhibitory concentrations (MIC) were identified after incubation for 24 h at 35 °C in aerobic conditions. Each test was repeated three times.
Effects of amoxicillin–clavulanic acid and levofloxacin on biofilms
Effect of antibiotics on bacterial biofilm was investigated in two frames: effects of subinhibitory concentrations of antibiotics were observed during the process of biofilm formation and effects of suprainhibitory concentrations (concentrations above the MIC) of antibiotics were observed on mature biofilm.
For biofilm assay bacteria were inoculated on tryptic soy agar (TSA; bioMérieux) and cultivated in aerobic conditions for 24 h at 35 °C. Bacterial suspensions of grown cultures were prepared in sterile saline (bioMérieux) and adjusted to density of 0.5 McFarland standard (approximately 108 CFU/ml), and then diluted to 106 CFU/ml.
Effects of subinhibitory concentrations of amoxicillin–clavulanic acid and levofloxacin on biofilm formation
Capacity of bacteria to form biofilm was investigated in 96-well microtiter plates in accordance with Stepanović et al. [20]. Amoxicillin–clavulanic acid and levofloxacin were prepared in fresh tryptic soy broth (TSB; bioMérieux) with the addition of 1% glucose in five serial dilutions (1/2× to 1/16× MIC), and 180 µl of each dilution was distributed in triplicates into microtiter plates. A 20 µl of previously prepared bacterial suspension was added to each well. Positive controls of each strain (bacteria in medium without antibiotics) were incubated under the same conditions. Negative controls for each plate were TSB medium solely, or with antibiotics. Plates were incubated for 24 h at 35 °C in aerobic conditions, decanted and rinsed gently three times with 300 µl of sterile phosphate-buffered saline (PBS; pH 7.2) for removal of planktonic bacterial cells. Fixation of biofilm was conducted on dry plates with 150 µl methanol per well for 20 min; plates were dried again and stained with 150 µl (per well) of 2% crystal violet (bioMérieux) for 15 min. Unbounded dye was thoroughly rinsed with water, plates were air dried and dye bound to the biofilm was released with 150 µl of 96% ethanol per well for 20 min. Optical density (OD) was measured at 570 nm using a microtiter plate reader (ICN Flow Titertek Multiscan Plus) and results were calculated according to Stepanović et al. [20]. Each assay was repeated three times. To calculate the category of biofilm formation, the cutoff optical density (ODc) was determined as three standard deviations above the mean OD of the negative control. According to the obtained results all tested strains were divided into four groups: OD ≤ ODc—category 0 (no biofilm producer); ODc < OD ≤ 2 × ODc—category 1 (weak biofilm producer, +); 2× ODc < OD ≤ 4× ODc—category 2 (moderate biofilm producer, ++); 4× ODc < OD—category 3 (strong biofilm producer, +++).
Effects of suprainhibitory concentrations of amoxicillin–clavulanic acid and levofloxacin on mature biofilm
To investigate the effects of amoxicillin–clavulanic acid and levofloxacin on mature biofilms, bacteria were cultivated in TSB medium in 96-well microtiter plates as previously described. After 72 h of incubation at 35 °C in aerobic conditions plates were decanted and rinsed gently three times with 300 µl of sterile PBS. Subsequently, mature biofilms were exposed to 4, 8, 16, 32, and 64 µg/ml of antibiotics (200 µl/well). Plates were incubated for additional 24 h at 35 °C in aerobic conditions, rinsed, fixed, and dyed as previously described, and the category of remained biofilm was calculated.
Statistical analysis
The data obtained in this study were analyzed in SPSS statistical program (PASW statistics for Windows, Version 18.0, Chicago: SPSS Inc. USA) using methods of descriptive statistics, Chi square test and Mann–Whitney U test.
Results
Out of the 48 patients who met the inclusion criteria and participated in the study, 27 were male (56.2%) and 21 female (43.8%), aged from 27 to 75 years (52.60 ± 13.03). Histopathological examination showed biofilms in 37 (77.1%) patients with CRSwNP. Bacterial strains were isolated in 42 (87.5%) patients: one microorganism in 34 (80.9%) and two microorganisms in 8 (19.1%).
S. epidermidis (34%) and S. aureus (28%) were the most commonly detected microorganisms in patients, followed by S. haemolyticus (12%), P. aeruginosa (8%), M. catarrhalis (6%), S. pneumoniae (6%), S. warneri (4%), and S. lugdunensis (2%).
Resistance profile of analyzed bacteria and MIC values for levofloxacin and amoxicillin–clavulanic acid are presented in Table 1.
Biofilm formation by clinical isolates of CRSwNP patients
The ability of clinical isolates to form biofilm is presented in Table 1. Most of the tested bacterial strains (92%) produced biofilm with different capacity: 27 (54%) strains were weak biofilm producers (category 1, +), 14 (28%) strains were moderate biofilm producers (category 2, ++) and 5 (10%) strains were strong biofilm producers (category 3, +++). Four (8%) strains had no capacity to produce biofilm (100% of S. pneumoniae isolates and 5.9% of S. epidermidis isolates).
S. aureus strains formed significantly voluminous biofilm compared to coagulase negative staphylococci (CoNS) (p < 0.01) and other isolated species (p < 0.05).
In eight patients with polymicrobial biofilms, different species of CoNS with weak biofilm-forming capacity (category 1, +) were detected: S. epidermidis and S. haemolyticus in four patients, S. epidermidis and S. warneri in three patients and S. haemolyticus and S. warneri in one patient.
Effects of subinhibitory concentrations of amoxicillin–clavulanic acid and levofloxacin on biofilm formation
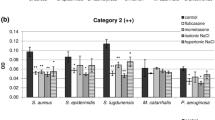
The effects of subinhibitory concentrations of amoxicillin–clavulanic acid and levofloxacin on biofilm formation were examined in four concentrations (1/2× to 1/16× MIC) and presented in Fig. 1. Amoxicillin–clavulanic acid expressed a significantly better inhibitory effect on biofilm formation compared to levofloxacin (p < 0.05).
Significant activity of amoxicillin–clavulanic acid on biofilm formation in all tested bacteria was evinced in 1/2×, 1/4×, and 1/8× MIC concentrations, where most of the strains reduced the capacity of biofilm formation (74, 78, and 70%, respectively).
Dose-dependent activity of amoxicillin–clavulanic acid on biofilm formation was observed only in S. aureus and CoNS with significant reduction in S. aureus strains that produced robust biofilm (p < 0.01). The effect of amoxicillin–clavulanic acid on biofilm formation by M. catarrhalis and P. aeruginosa in all tested concentration was inhibitory but not dose dependent (Fig. 1a).
Inhibitory effect of levofloxacin on biofilm formation was significantly better on M. catarrhalis (in 1/2× to 1/8× MIC concentrations, p < 0.05) and P. aeruginosa (in 1/2× and 1/4× MIC concentrations; p < 0.01 and p < 0.05) compared to staphylococci (Fig. 1b).
Effects of suprainhibitory concentrations of amoxicillin–clavulanic acid and levofloxacin on mature biofilm
The effects of both antibiotics on mature biofilms were tested in five concentrations: 4, 8, 16, 32, and 64 µg/ml. Levofloxacin expressed better inhibitory effect on biofilm eradication compared to amoxicillin–clavulanic acid (p < 0.05).
Amoxicillin–clavulanic acid and levofloxacin contributed to the eradication of mature biofilms of staphylococci, with more pronounced effect on S. aureus strains (p < 0.01; p < 0.01) (Fig. 2a, c). The effect of levofloxacin on eradication of staphylococcal biofilm was more noticeable, compared to the effects of amoxicillin–clavulanic acid (p < 0.01) (Fig. 2c).
Both antibiotics had no eradication activity on M. catarrhalis mature biofilm, while suprainhibitory concentrations of levofloxacin eradicated biofilms only in 25% of P. aeruginosa strains, regardless to the applied dose (Fig. 2b, d).
Discussion
Biofilms in sinonasal tract are present both in healthy individuals and CRS patients. There is evidence that the presence of polymicrobial biofilm is significant for health and impaired diversity with increased abundance is associated with chronic inflammation and poor healing [21]. Similar results were obtained in our study, with the presence of a single bacterial species in most of CRSwNP patients with significant biofilm-forming capacity. Furthermore, polymicrobial biofilms consisting of two different species of low-virulence CoNS with a weak biofilm-forming capacity were detected only in a small number of patients in the study. Although a polymicrobial biofilm may also be formed, each bacterial species forms its own microcolony aggregate rather than mixing with each other, hence the effects of external environment (pH, temperature, antibiotics, etc.) act on a single species [22]. Therefore, we investigated biofilm-forming capacity of an individual isolate.
In accordance with the results of other investigations [23], the most common isolates in CRS biofilms were S. aureus and S. epidermidis. Although the general opinion that pathogenicity of some low-virulence nasal colonizing bacteria, such as S. epidermidis, is questionable [24], and that CoNS are not considered respiratory tract pathogens by the infectious disease specialists [10], our findings confirm their role in biofilms of CRSwNP patients.
The role of antibiotics in treatment of CRS biofilms has not been sufficiently investigated [15,16,17]. Therefore, we designed a study analyzing the effects of most commonly used antibiotics in treatment of CRS, amoxicillin–clavulanic acid, and levofloxacin, at the various stages of biofilm formation by different clinical strains isolated from patients with CRSwNP. Besides being most commonly used, these two antibiotics also target different microbial structures, have a dissimilar structure and display different pharmacokinetics and pharmacodynamics. Amoxicillin–clavulanic acid represents an antibiotic that is predominantly located in extracellular fluid and, like other beta-lactams, needs to maintain concentrations above the MIC for at least 40–60% of the interval time between administrations to be potentially effective [25, 26]. Dinis et al. conducted a randomized, open, single-dose, sinus tissue pharmacokinetic study with oral dose of 875/125 mg amoxicillin/clavulanic acid 2–4 h before CRS surgery and concluded that amoxicillin moderately concentrate in sinus tissue (uncinate processus, bulla ethmoidalis, maxillary sinus mucosa or cysts, middle turbinate, posterior ethmoid, sphenoid, and frontal sinus) during 4 h after administration of antibiotics, while clavulanic acid remains at the close level to plasma concentration during the same time [27]. The concentrations of amoxicillin in blood ranged from 0.10 to 5.59 mg/ml, and in the sinuses from 1.04 to 7.58 mg/g. Clavulanic acid concentration ranged from 0.04 to 2.43 mg/ml in plasma to 0.00 to 2.90 mg/g in sinonasal tissue. Similar results were obtained by Passali et al. [25], with the tissue concentrations of both amoxicillin and clavulanic acid above MIC for the most frequent causative pathogens of sinus bacterial infections during the 2–6 h after the administration of antibiotic. Authors recommended therapeutic dosage of 1 g twice a day even in patients suffering from acute episodes of chronic rhinosinusitis.
In contrast with beta-lactams, which remain predominantly extracellular, fluoroquinolones exhibit concentration dependent bactericidal activity, with optimal peak-MIC ratio of 8–10 to achieve optimal clinical outcome, or eradication of bacteria and prevention of resistance [28, 29]. Levofloxacin is a fluoroquinolone with rapid and high absorption after oral administration, with 99% of bioavailability and relatively long half-life (7–9 h). Plasma concentrations achieved with once daily administration of levofloxacin provide sufficient AUC to achieve AUC:MIC ratios optimal for clinical cure of a broad spectrum of bacteria. Mean pharmacokinetic parameters of levofloxacin in healthy adult subjects after oral administration of 500 mg are: Cmax, (peak plasma concentration): 5.7–6.6 mg/l; Tmax (time to Cmax): 1.1–1.2 h and AUC0–24 (area under the plasma concentration–time curve from time 0–24 h): 48–54 mg h/l [30, 31]. In contrast to other antibacterial agents with poor tissue penetration (penicillins, macrolides, etc.), fluoroquinolones have excellent pharmacokinetic properties, with enhanced tissue penetration and selective acumulation in inflammed tissues. Several studies of fluoroquinolones PK in CRS have demonstrated that tissue concentrations of antibiotics in patients with CRS were 2–3 times higher than concomitant serum concentrations over a 3–36 h period. Pea et al. demonstrated that after a single 500 mg oral levofloxacin, median plasma concentrations were 0.67 mg/l at 1 h, 3.45 mg/l at 2 h, 1.88 mg/l at 3 h, and median mucosal concentrations were 0.96 mg/l at 1 h, 2.50 mg/l at 2 h and 5.84 mg/l at 3 h [32]. Average paranasal sinuses mucosa-to-plasma ratios were increasing during time from 1.46 at 1 h, to 1.81 at 2 h and to 2.56 at 3 h [32]. Similar paranasal sinuses mucosa-to-plasma ratios were achieved during 36 h period after a last dose of 400 mg moxifloxacine (administred daily for 5 days) in maxillary sinus mucosa, anterior ethmoid mucosa and nasal polypes [26], and ciprofloxacin over a 12 h period [33]. Optimal pharmacokinetic profile and acumulation in sinus tissue make levofloxacin suitable for once a day administration of 500 mg in patients with CRSwNP.
Although it is unlikely to administrate subinhibitory concentrations of antibiotics in clinical settings, these concentrations can be reached at the end of the dose-interval or if the doses were not taken adequately. It is also likely that subinhibitory concentrations are present in the deeper layers of biofilm, both due to altered diffusion of antibiotics and interaction of antibiotics with EPS. Either way, even if doses are administrated properly, the effective concentration of antibiotic in biofilm most likely does not correspond to the plasma and tissue concentration. Subinhibitory concentrations of both tested antibiotics inhibited early stages of biofilm formation in a dose-dependent manner with a better activity of amoxicillin–clavulanic acid compared to levofloxacin. Significant activity of amoxicillin–clavulanic acid against staphylococcal species and M. catarrhalis was detected in concentrations ranging from 1/2× to 1/8× MIC. The inhibitory effects of amoxicillin–clavulanic acid were probably due to direct bactericidal effect, since it is well known that beta-lactams are most active against actively growing bacteria [34]. Sedlacek and Walker confirmed the reduction of viable cell numbers after treatment of bacterial biofilms with amoxicillin–clavulanic acid [35]. Moderate activity against P. aeruginosa could be a result of lower susceptibility of the bacterium to beta-lactams and altered diffusion of antibiotic due to the extensive EPS production. On the other hand, only concentration of levofloxacin corresponding to 1/2× MIC significantly reduced biofilm formation in all tested bacterial species. Acting predominantly as an intracellular antibiotic, levofloxacin has reduced activities of lower concentrations probably caused by impaired diffusion through biofilm and interaction of antibiotic with EPS.
In adequate dose intervals concentrations above MIC (i.e., suprainhibitory concentrations) are achieved at the site of the infection. Tissue levels of both amoxicillin–clavulanic acid and levofloxacin during first hours after a single-dose administration are higher than the MIC for the most upper respiratory bacterial pathogens [25, 32]. Suprainhibitory concentrations of antibiotics tested in the study expressed a lower biofilm-eradication activity, as previously reported for other antibiotics. The thickness of biofilm and the density of extracellular polymer matrix are the most relevant factors that limit the diffusion of antibiotics through bacterial biofilms and decrease the antibacterial activity of those substances. However, staphylococcal mature biofilms were more susceptible, especially to the effect of levofloxacin. Antibiofilm effect of fluoroquinolones on mature biofilm is probably a result of altered adhesion of bacteria, disruption of EPS in biofilm after fluoroquinolone-induced activation or release of enzymes [36] and bactericidal effects of fluoroquinolones against bacteria in stationary phase of growth [37]. Fluoroquinolones also reduce the synthesis of bacterial nucleic acids, consequently reducing the amount of extracellular DNA, one of the most important compounds that increase the density and strength of EPS. After the disruption of EPS, bacteria released from biofilm are killed with the same mechanisms as their planktonic forms. Reduced activity of beta-lactams on biofilm of other bacterial species corresponds to the results of other investigators [38, 39]. It is most likely that amoxicillin–clavulanic acid has a low diffusion rate through dense matrixes of mature biofilms. In mature biofilms, the amount of extracellular fluid reduces, with a consequent decrease in beta-lactam concentration, since these antibiotics are predominantly localized extracellularily. Conversely, the diffusion of fluoroquinolones is not impaired due to their moderate lipophylicity. Similarly, in chronic infections the penetration of beta-lactams is diminished by altered blood flow and reduced volume of extracellular fluid [32], while the penetration of fluoroquinolones into sinus tissues is minimally affected by the local inflammatory status [24, 32]. Besides, beta-lactams predominantly aim actively growing cells, relatively sparse in mature biofilms compared to their dormant forms.
The results of our study demonstrated that antibiofilm effects of amoxicillin–clavulanic acid and levofloxacin depend on bacterial species and bacterial biomass. Future studies need to establish molecular mechanisms of these findings.
Conclusions
In conclusion, amoxicillin–clavulanic acid and levofloxacin express a better antibiofilm activity at early stages of biofilm formation than in eradication of mature biofilms. In empiric treatment of CRSwNP patients, amoxicillin–clavulanic acid could be recommended as first-line antibiofilm therapy choice.
References
DeConde AS, Soler ZM (2016) Chronic rhinosinusitis: epidemiology and burden of disease. Am J Rhinol Allergy 30:134–139
Lam K, Schleimer R, Kern RC (2015) The etiology and pathogenesis of chronic rhinosinusitis: a review of current hypotheses. Curr Allergy Asthma Rep 15:41
Lee S, Lane AP (2011) Chronic rhinosinusitis as a multifactorial inflammatory disorder. Curr Infect Dis Rep 13:159–168
Cryer J, Schipor I, Perloff JR, Palmer JN (2004) Evidence of bacterial biofilms in human chronic sinusitis. ORL J Otorhinolaryngol Relat Spec 66:155–158
Bendouah Z, Barbeau J, Hamad WA, Desrosiers M (2006) Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg 134:991–996
Psaltis AJ, Ha KR, Beule AG et al (2007) Confocal scanning laser microscopy evidence of biofilms in patients with chronic rhinosinusitis. Laryngoscope 117:1302–1306
Singh R, Ray P, Das A, Sharma M (2010) Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother 65:1955–1958
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Stewart PS (2002) Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol 292:107–113
Barshak MB, Durand ML (2017) The role of infection and antibiotics in chronic rhinosinusitis. Laryngoscope Investig Otolaryngol 2:36–42
Slavin RG, Spector SL, Bernstein IL (2005) The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol 116:S13–S47
Cain RB, Lal D (2013) Update on the management of chronic rhinosinusitis. Infect Drug Resist 6:1–14
Russell PT, Bekeny JR (2014) Oral antibiotics and the management of chronic sinusitis: what do we know? Curr Opin Otolaryngol Head Neck Surg 22:22–26
Hamilos DL (2000) Chronic sinusitis. J Allergy Clin Immunol 106:213–227
Korkmaz H, Ocal B, Tatar EC et al (2014) Biofilms in chronic rhinosinusitis with polyps: is eradication possible? Eur Arch Otorhinolaryngol 1:2695–2702
Desrosiers M, Bendouah Z, Barbeau J (2007) Effectiveness of topical antibiotics on Staphylococcus aureus biofilm in vitro. Am J Rhinol 21:149–153
Ha KR, Psaltis AJ, Butcher AR et al (2008) In vitro activity of mupirocin on clinical isolates of Staphylococcus aureus and its potential implications in chronic rhinosinusitis. Laryngoscope 118:535–540
Stammberger H (1999) Surgical treatment of nasal polyps: past, present, and future. Allergy 54(Suppl 53):7–11
Hochstim CJ, Choi JY, Lowe D et al (2010) Biofilm detection with hematoxylin-eosin staining. Arch Otolaryngol Head Neck Surg 136:453–456
Stepanović S, Vuković D, Hola V et al (2007) Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115:891–899
Gontcharova V, Youn E, Sun Y et al (2010) A comparison of bacterial composition in diabetic ulcers and contralateral intact skin. Open Microbiol J 4:8–19
Burmolle M, Thomsen TR, Fazli M et al (2010) Biofilms in chronic infections-a matter of opportunity-monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol 59:324–336
Boase S, Foreman A, Cleland E et al (2013) The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect Dis 13:210
Brook I (2016) Microbiology of chonic rhinosinusitis. Eur J Clin Microbiol Infect Dis 35:1059–1068
Passali D, Mazzei T, Novelli A et al (2001) Amoxicillin/clavulanate in chronic rhinosinusitis: tissue and serum distribution. Acta Otorhinolaryngol Belg 55:259–264
Gehanno P, Darantiere S, Dubreuil C et al (2002) A prospective, multicentre study of moxifloxacin concentrations in the sinus mucosa tissue of patients undergoing elective surgery of the sinus. J Antimicrob Chemother 49:821–826
Dinis PB, Monteiro MC, Martins ML, Silva N, Gomes A (2000) Sinus tissue pharmacokinetics after oral administration of amoxicillin/clavulanic acid. Laryngoscope 110:1050–1055
Preston SL, Drusano GL, Berman AL et al (1998) Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125–129
Aminimanizani A, Beringer P, Jelliffe R (2001) Comparative pharmacokinetics and pharmacodynamics of the newer fluoroquinolone antibacterials. Clin Pharmacokinet 40:169–187
Chien SC, Rogge MC, Gisclon LG et al (1997) Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother 41:2256–2260
Child J, Mortiboy D, Andrews JM et al (1995) Open-label crossover study to determine pharmacokinetics and penetration of two dose regimens of levofloxacin into inflammatory fluid. Antimicrob Agents Chemother 39:2749–2751
Pea F, Marioni G, Pavan F et al (2007) Penetration of levofloxacin into paranasal sinuses mucosa of patients with chronic rhinosinusitis after a single 500 mg oral dose. Pharmacol Res 55:38–41
Gehanno P, Farinotti R, Buffe P, Cohen B, Cudennec Y, Sterkers O (1992) Penetration of ciprofloxacin into middle ear and sinus mucosa after repeated oral administration. In: Program and abstracts of the fifth international congress for infectious diseases, Nairobi, Kenya, 1992. Abstract 516. International Society for Infectious Diseases, Boston, p 147
Gilbert P, Brown MR (1998) Biofilms and beta-lactams activity. J Antimicrob Chemother 41:571–572
Sedlacek MJ, Walker C (2007) Antibiotic resistance in an in vitro subgingival biofilm model. Oral Microbiol Immunol 22(5):333–339
Yassien M, Khardori N (2001) Interaction between biofilms formed by Staphylococcus epidermidis and quinolones. Diagn Microbiol lnfect Dis 40:79–89
Eng RHK, Padberg FT, Smith SM et al (1991) Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother 35:1824–1828
Drago L, Mattina R, Legnani D et al (2011) Modulation of biofilm of strains isolated from patients with chronic obstructive pulmonary disease by levofloxacin, moxifloxacin, ciprofloxacin, amoxicillin/clavulanic acid and ceftriaxone. Int J Immunopalhol Pharmacol 24(4):1027–1035
Maestre JR, Mateo M, Mendez ML et al (2010) In vitro interference of beta-lactams with biofilm development by prevalent community respiratory tract isolates. Int J Antimicrob Agents 35:274–277
Acknowledgements
We would like to thank Dr Neda Konstantinovic for her help in preparing this study. This work was supported by the Ministry of Education, Science and Technological Development of Serbia, Grant no. ON175039, http://www.mpn.gov.rs/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Božić, D.D., Pavlović, B., Milovanović, J. et al. Antibiofilm effects of amoxicillin–clavulanic acid and levofloxacin in patients with chronic rhinosinusitis with nasal polyposis. Eur Arch Otorhinolaryngol 275, 2051–2059 (2018). https://doi.org/10.1007/s00405-018-5049-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-5049-6