Abstract
Tobacco smoking was one of the risk factors for upper aerodigestive tract cancer, but exclusive quantification of the impact of cigarette smoking on laryngeal cancer had not been investigated. A meta-analysis of researches that had reported quantitative estimates of cigarette smoking and risk of laryngeal cancer by March 2016 was performed. Pooled estimates of relative risks and their 95% confidence intervals were obtained and summarized. Sensitivity analysis and subgroup analysis were implemented to find out sources of research heterogeneity and the effect of potential confounders. Publication bias was investigated and corrected if found to be present through Egger’s and Begg’s test, and trim and fill algorithm. Thirty researches based on a total of 14,292 cases from three cohort and fifteen case–control studies were included and pooled estimate for the correlation between cigarette smoking and the risk of laryngeal cancer was 7.01 (95% confidence interval 5.56–8.85), with moderate heterogeneity across the researches (I 2 = 56.7%, p = 0.002). The RRs were 5.04 (95% CI 3.09–8.22) for cohort studies (p = 0.121), 7.59 (95% CI 5.86–9.82) for case–control studies (p = 0.005). The risk kept elevated within the first fifteen years of quitting smoking(RR 3.62, 95% CI 1.88–7.00) but dropped in the 16 years and more after smoking cessation(RR 1.88, 95% CI 1.16–3.05). Individuals who smoked with 40 or more pack-years had nine times the risk of laryngeal cancer(RR 9.14; 95% CI 6.24–13.39). Subjects who smoked 30 or more cigarettes a day had sevenfolds the risk of laryngeal cancer (RR 7.02; 95% CI 4.47–11.02) and who smoked 40 or more years had five times the risk versus never smokers (RR 5.76; 95% CI 3.69–8.99). Evidence of publication bias was not detected for the correlation between current cigarette smoking and risk of laryngeal cancer (p = 0.225 with Begg’s test, p = 0.317 with Egger’s test). The results demonstrated strong correlation referring to dose–response and time–response between cigarette smoking and risk of laryngeal cancer for both men and women. The probability of developing laryngeal cancer was decreased by quitting smoking, particularly among former cigarette smokers who had stopped smoking for 15 or more years. The subgroup analysis demonstrated that study type influenced the RRs estimates of the studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco smoking and alcohol consumption were considered as lifestyle-related risk factors correlated with increased risk of upper aerodigestive tract cancer (UADTC) with synergistic effect [20, 22]. The carcinogenic effects of tobacco and alcohol were identified to be multiplicative on the relative risk magnitude in a meta-regression [41]. Tobacco appeared to possess a much stronger impact on the larynx than on the other aerodigestive sites [22]. The heterogeneity of the pooled estimates was present among researches with or without adjustment for alcohol consumption in a meta-analysis based on information reported in the International Agency for Research on Cancer (IARC) Monograph on ‘‘Tobacco Smoke and Involuntary Smoking’’ [15]. Lung cancer (RR = 8.96; 95% CI 6.73–12.11) and laryngeal cancer (RR = 6.98; 95% CI 3.14–15.52) showed the highest relative risks for current smokers. Evidence from either observational or experimental researches had presented a positive correlation between cigarette smoking and risk of incidence or mortality of laryngeal cancer [19].
The association between cigarette smoking and laryngeal cancer risk had been reviewed in several publications [38]. The pooled estimate of 120 studies for the association between current smoking and risk of UADTC was 3.47 (95% CI 3.06–3.92) in a meta-analysis, with the strength of the association being significantly stronger for laryngeal cancer (RR 9.07; 95% CI 6.33–13.0) versus other subtypes of UADTC [30]. A strong dose–response association between the number of cigarettes smoked per day and the risk of laryngeal cancer was observed both before and after adjustment for publication bias [30]. A pooled analysis of case–control studies indicated that greater number of cigarettes smoked per day for a shorter duration was less deleterious than fewer cigarettes/day for a longer duration and the greater risk of laryngeal cancer derived from different numbers of cigarettes smoked per day effects and not pack-years [27].
However, few studies examined the association between cigarette smoking status, pack-years, frequency, duration and cessation of cigarette smoking and laryngeal cancer risk through a meta-analysis. In this study, evidence from published observational researches regarding the association between cigarette smoking without alcohol consumption and laryngeal cancer risk were searched and summarized quantitatively with a meta-analytic approach. The study was carried out and written following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.
Materials and methods
Study identification, eligibility and exclusion criteria
All published epidemiologically observational studies which surveyed the correlation between cigarette smoking and risk of laryngeal cancer were included in the meta-analysis if they satisfied all of the following criteria: (1) possessed original data expressed as pooled estimates of relative risks (RRs) and their 95% confidence intervals(CI) from case–control studies, nested case–control studies or cohort studies; (2) the primary outcome of cases was clearly diagnosed as the cancer of the larynx; (3) had a quantitative estimate of the association between cigarette smoking status, pack-years, frequency, duration and cessation of cigarette smoking and laryngeal cancer risk, expressed as hazard ratios(HRs for cohort studies) and odds ratios (ORs for case–control studies) for each category of cigarette smokers versus never smokers; (4) risk estimates were necessarily adjusted for selected confounding factors or covariates; (5)were published in English or Chinese language; (6) subjects and controls did not use alcohol, and (7) were published up to March 2016. If a study arised in various articles, data with the largest sample size published in the most recent years were included for the meta-analysis.
The pooled analysis, reviews, meta-analysis, duplicated, unpublished or non-original researches, cross-sectional and articles in cell lines or animals were excluded. In addition, studies were excluded from this meta-analysis if they met any one of the following criteria: (1) laryngeal cancer cases were concurrently suffered from viral infection, occupational factors exposure, obesity, and other diseases such as diabetes, concurrent or secondary cancers; (2) other forms of tobacco-related consumption, including tobacco chewing, chewing of betel leaf with tobacco, smokeless tobacco, water pipes, cigar, cigarillos or bidi smoking, pipe smoking, marijuana smoking, electronic cigarette, involuntary smoking exposure at home or at work; (3) cases with alcohol consumption, betel quid or areca nut chewing, and a family of malignancy; (4) cases were previously under radiotherapy, chemotherapy and surgical treatment; (5) not reporting a quantitative estimate of the association expressed as pooled estimates of ORs or RRs and their 95% CI.
Search strategy
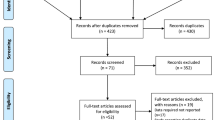
Such databases as Up to Date, ACP journal club, Medline, PubMed Clinical Queries, Sumsearch, Ovid, Tripdatabase, Cochrane Library (CDSR, CCTR, DARE), Embase, Chinese Biomedical Database(CBM), China National Knowledge Infrastructure(CNKI), CqVip, and Wanfang were completely searched from their establishment to March 2016 for gathering relevant medical literature. Data retrievals were limited to the English and Chinese language and done in parallel by Chen Chen and Jing Jing Zuo. Free term searching was adopted given that observational studies were not indexed as subject terms in the search engines. The key words and free term used were as follows: (“cigarette smoking” OR “tobacco smoking”) AND (“laryngeal cancer” OR “head and neck cancer”) AND (etiology OR “risk factor”) AND (“case–control study” OR “cohort study”). The articles reporting data on the association between cigarette smoking and risk of laryngeal cancer incidence were selected, with no other and previous diagnosis of cancer at any site of body. Figure 1 shows the flowchart for screening of articles. The reference lists of all papers of interest were examined to get other related publications and the corresponding authors were contacted to confirm data extraction when data were incomplete or uncertain data extraction and methodological quality assessment.
Baseline information of available data were drawn and compared by two reviewers separately using the same criteria. The disagreements about results were resolved through consensus. Information on country, design, sample size, and sex of studies, the variables that the study results were controlled, the RRs and their corresponding 95% CIs for cigarette smoking status and each category of the pack-years, frequency, duration and cessation of cigarette smoking were extracted. The maximal results adjusted were adopted from the studies which reported both crude and adjusted RRs and 95% CIs, while the papers with unadjusted estimates only were ruled out. The more updated article was contained when two or more papers offered results from the same research. The countries or districts of included studies were divided into Europe–America and non-Europe–America, while amounts of sample sizes were categorized as big sample size (≥300) and small sample size (<300). Quality evaluation of included studies was conducted following the Newcastle–Ottawa Scale (NOS) criteria which possessed four items for selection, two items for comparability, and three items for evaluation of outcomes or exposures. A maximum of nine points was designated to each research, while scores of 7–9 and 0–6 were considered as high quality and non-high quality separately.
Statistical analysis
The adjusted relative risks (RRs) and their corresponding 95% CI from each eligible research were combined for the meta-analysis, weighted by the inverse of their variance, and p < 0.05 was considered statistically significant. The pooled RRs and 95% CIs between laryngeal cancer and cigarette smoking were estimated by meta-analysis and offered with forest plots for each category of cigarette smokers versus never smokers. Meanwhile, the association between laryngeal cancer risk and the pack-years, frequency, duration and cessation of cigarette smoking was carried out separately. Statistical heterogeneity among studies was assessed with I 2 statistic and the results with p < 0.10 were identified as heterogeneous [21]. The results of I 2 statistic with values of 25, 50, and 75% represented low, moderate, and high degrees of heterogeneity, respectively. Heterogeneity was regarded statistically significant when I 2 > 50% and p < 0.1, then sensitivity analysis was conducted by excluding one study once each time and investigating the impact of each individual research on the overall relative risk. Subgroup analysis was implemented to find out sources of research heterogeneity and the effect of potential confounding factors such as country, design, sample size, and sex of studies.
Heterogeneity was not recognized statistically significant when I 2 < 50% and p > 0.1 and fixed effects model was adopted. Instead, random effects model that took variation both between and within researches into account was then chosen to analyze the data. Publication bias was assessed through examination of Egger’s test and Begg’s test [8] (significant at p < 0.1). Trim and fill algorithm was conducted to check and correct for the asymmetry of funnel plot from publication bias possibly [35]. All statistical analyses were conducted with the Stata 11 (Stata Corporation, College Station, TX, USA) statistical software and their tests are two-sided.
Results
A total of four cohort studies and 26 case–control studies, with information about 14,292 cases of laryngeal cancers and 45,579 individuals as control, were included in the meta-analysis [1–7, 9–14, 17, 18, 23–26, 28, 31–34, 36, 37, 39, 40, 42, 43]. The summary features of included studies with risk estimates and corresponding 95% CI for cigarette smoking and laryngeal cancer are showed in Table 1. The majority of the researches were from Europe (n = 12), America (n = 6), Asia (n = 11), and Africa (n = 1). Table 2 gave the risk estimates and their corresponding 95% CIs for each category of the pack-years, frequency, duration and cessation of cigarette smoking and laryngeal cancer.
Quality assessment
Quality evaluation of all included studies was carried out using the Newcastle–Ottawa Quality Assessment Scale for case–control and cohort studies (Table 1). Among case–control studies, 16 studies (accounted for 61.5%) were considered as high quality and only Pacella-Norman et al. [32] scored the lowest, while all cohort studies scored highest independently. Most studies were of good quality with no evidence of selection bias, good outcome assessment of cohort studies, and with good comparability of the exposed and unexposed groups of case–control studies.
Cigarette smoking status and the risk of laryngeal cancer
Twenty-four articles that presented the correlation between cigarette smoking and risk of laryngeal cancer were identified and their pooled estimate was 6.62 (95% CI 5.26–8.35) through a meta-analytic approach, with significant heterogeneity between the studies (I 2 = 83.5%, p = 0.000). Sensitivity analysis via excluding every study was conducted to find out six related data of studies [8, 9, 12, 16, 20, 26] as the source of heterogeneity between study estimates (Fig. 7). The subsequent analyses were limited to studies (n = 18) involving 3279 laryngeal cancers events and 11,252 participants. The pooled estimate of the eighteen studies for the correlation between current smoking and risk of laryngeal cancer was 7.01 (95% CI 5.56–8.85), with moderate heterogeneity across the studies (I 2 = 56.7%, p = 0.002; Table 2). The RRs were 5.04 (95% CI 3.09–8.22) for cohort studies (p = 0.121), 7.59 (95% CI 5.86–9.82) for case–control studies (p = 0.005; Fig. 2). Sensitivity analysis by omitting every research revealed similar outcomes, suggesting the robustness of the results.
Cigarette smoking status and the risk of laryngeal cancer in a meta-analysis. Black squares represent point estimates and horizontal lines indicate 95% CIs for the observed effect in each research. A white diamond represents a pooled estimate and 95% CI for meta-analysis. ID1 cohort study, ID2 case–control study, RR relative risk, CI confidence interval
A great amount of the heterogeneity was interpreted by differences between the two types of sample size using a subgroup analysis method, with the strength of the association being notably stronger for small sample size group(RR 7.19; 95% CI 5.31–9.74) versus large sample size group (RR 6.46; 95% CI 5.05–8.27). The research heterogeneity was much lower in studies with large sample size (I 2 = 2.5%, p = 0.359), compared with studies with small sample size (I 2 = 62.2%, p = 0.001; Table 2). The different heterogeneity between the two types of sample size once again confirmed that the studies with small sample size, compared with those with large sample size, had lower quality statistically and were prone to show a higher effect estimate leading to bias.
Geographical discrepancies of the research populations were another source of heterogeneity between study estimates, with the extent of the association being obviously greater among populations from Europe and America group compared with non-Europe and America group: the RRs were 7.89 (95% CI 5.77–10.77) and 6.64 (95% CI 4.83–9.12) separately. The study heterogeneity was much lower in studies from Europe and America group (I 2 = 24.1%, p = 0.237) compared with that in studies from non-Europe and America group (I 2 = 87.2%, p = 0.001), suggesting inferior quality of the latter studies relatively.
Information on sex of studies was extracted and moderate heterogeneity was shown in one sex group using a subgroup analysis method (I 2 = 25.3%, p = 0.227). Comparing with the size of heterogeneity in geographical discrepancies or sample size, the differences in sexes were not the primary source of heterogeneity between study estimates.
Pack-years of cigarette smoking and risk of laryngeal cancer
Twenty-seven studies reported the association between pack-years of cigarette smoking and risk of laryngeal cancer and their pooled risk estimate was 4.31 (95% CI 3.22–5.77), with prominent heterogeneity between the studies (I 2 = 95.2%, p = 0.000). Sensitivity analysis through excluding every study was adopted to identify five related data of studies [18, 34] as the source of heterogeneity between study estimates.
A total of 22 articles had presented four proprietary forms for pack-years of smoking: never smokers, <20 pack-years, 20–39 pack-years, and ≥40 pack-years, including 2367 laryngeal cancers events and 7613 participants. The summary estimate of the 22 identified studies for the association between pack-years of cigarette smoking and risk of laryngeal cancer was 4.61 (95% CI 3.44–6.18), with moderate heterogeneity between the studies (I 2 = 62.7%, p = 0.000; Fig. 3) and no significant heterogeneity between the four subtypes of studies categorized by pack-years (Table 2). The RRs were 3.03 (95% CI 1.88–4.89) for cohort studies (p = 0.326), 4.97 (95% CI 3.58–6.92) for case–control studies (p < 0.001).
Pack-years of cigarette smoking and risk of laryngeal cancer in a meta-analysis (for definitions of the squares, lines, and diamond; see Fig. 1). ID1 0–19 pack-years, ID2 20–39 pack-years, ID3 ≥40 pack-years, RR relative risk, CI confidence interval
The risk of laryngeal cancer exacerbated gradually as the number of pack-years of cigarette smoking increased. Individuals who smoked with 40 or more pack-years had ninefold the risk of laryngeal cancer versus never smokers (RR 9.14; 95% CI 6.24–13.39). Sensitivity analysis by deleting every research showed similar results, indicating the robustness of the outcomes. The moderate heterogeneity was explained by differences between the designs of study, with the strongpoint of the association being stronger for case–control group (RR 4.97; 95% CI 3.58–6.92) versus cohort group (RR 3.03; 95% CI 1.88–4.89). The study heterogeneity was much lower in cohort group (I 2 = 10.7%, p = 0.326; Table 2), compared with that in case–control group (I 2 = 65.2%, p = 0.000). The outcomes of heterogeneity test demonstrate that the results of cohort studies, which had adjusted for major confounding factors, were more reliable than that of retrospective case–control studies which were more subject to bias.
Frequency of cigarette smoking and risk of laryngeal cancer
Thirty studies had investigated four specific types for daily cigarette consumption: never smokers, <20 cigarettes per day, 20–29 cigarettes per day, and ≥30 cigarettes per day. The pooled estimate of these articles that presented the association between frequency of cigarette smoking and risk of laryngeal cancer was 6.34 (95% CI 4.23–9.48), with noteworthy heterogeneity between the studies (I 2 = 86.5%, p = 0.000). Sensitivity analysis by eliminating every research identified nine related data of [9, 12, 16, 20, 26] studies as the source of heterogeneity between study estimates.
The resulting analyses were confined to twenty-one studies involving 2814 laryngeal cancer cases and 9636 controls. The pooled estimate of these studies for the association between frequency of cigarette smoking and risk of laryngeal cancer was 5.82 (95% CI 4.47–7.58), with medium heterogeneity between the studies (I 2 = 49.9%, p = 0.005; Fig. 4). The RRs were 3.65 (95% CI 2.40–5.54) for cohort studies (p = 0.523), 6.53 (95% CI 4.85–8.78) for case–control studies (p = 0.008). A substantial dose–response correlation between the number of cigarettes smoked per day and risk of laryngeal cancer was surveyed, such that the risk remained high for smoking <30 cigarettes a day and individuals who smoked 30 or more cigarettes a day had seven times the risk of laryngeal cancer versus never smokers (RR 7.02; 95% CI 4.47–11.02; Table 2). Sensitivity analysis by omitting every research revealed similar results, showing the robustness of the outcomes.
Frequency of cigarette smoking and risk of laryngeal cancer in a meta-analysis (for definitions of the squares, lines, and diamond; see Fig. 1). ID1 0–19 cigarettes per day, ID2 20–29 cigarettes per day, ID3 ≥30 cigarettes per day, RR relative risk, CI confidence interval
A large amount of the heterogeneity was interpreted by differences between continents adopting a subgroup analysis method, with the strength of the association being notably stronger for non-Europe and America group (RR 6.48; 95% CI 4.72–8.89) versus Europe and America group (RR 5.72; 95% CI 4.04–8.10). The study heterogeneity was much lower in non-Europe and America group (I 2 = 0.0%, p = 0.728), compared with that in Europe and America group (I 2 = 59.2%, p = 0.001; Table 2). Discrepancies between the design of studies were the other source of heterogeneity between study estimates, with the dimension of the association being stronger among populations from case–control group compared with cohort group: the RRs were 6.53 (95% CI 4.85–8.78) and 3.65 (95% CI 2.40–5.54) severally. The study heterogeneity was relatively lower in cohort group (I 2 = 0.0%, p = 0.523), compared with that in case–control group (I 2 = 51.0%, p = 0.008).
Duration of cigarette smoking and risk of laryngeal cancer
Twenty-nine researches presented the association between duration of cigarette smoking and risk of laryngeal cancer and their summary estimate by meta-analysis was 4.52 (95% CI 3.21–6.37), with significant heterogeneity between the studies (I 2 = 84%, p = 0.000). Sensitivity analysis by omitting every research was used to find out ten related data of studies [8, 9, 12, 16] as the source of heterogeneity between study estimates. The final analyses were restrained to studies (n = 19) involving including 2699 laryngeal cancer cases and 9669 controls. The pooled estimate of the 19 reports for the association between duration of cigarette smoking and risk of laryngeal cancer was 4.27 (95% CI 3.62–5.05; Table 2), with slight heterogeneity between the studies (I 2 = 26.9%, p = 0.136, fixed effects model used; Fig. 5). The RRs were 2.87 (95% CI 2.09–3.96) for cohort studies (p = 0.560), 4.95 (95% CI 4.07–6.01) for case–control studies (p = 0.398).
Duration of cigarette smoking and risk of laryngeal cancer in a meta-analysis (for definitions of the squares, lines, and diamond; see Fig. 1). Overall relative risk calculated with fixed effects model. ID1 0–19 years, ID2 20–29 years, ID3 30–39 years, ID4 ≥40 years, RR relative risk, CI confidence interval
A strong time–response correlation between duration of cigarette smoking and the risk of laryngeal cancer was indicated, such that the risk kept raised within 40 years and individuals who smoked 40 or more years had fivefold the risk of laryngeal cancer versus never smokers (RR 5.76; 95% CI 3.69–8.99; Table 2). Sensitivity analysis by excluding every research indicated similar outcomes, showing the robustness of the outcomes. Differences between the published years of studies might be the source of heterogeneity between study estimates through subgroup analysis, with the extent of the correlation being greater among studies published before 2006 compared with studies published after 2006: the RRs were 4.50 (95% CI 3.71–5.45) and 3.67 (95% CI 2.64–5.12) separately. The study heterogeneity was obviously lower in studies published after 2006 (I 2 = 0.0%, p = 0.524), compared with that in studies published before 2006 (I 2 = 42.5%, p = 0.066).
Smoking cessation and risk of laryngeal cancer in former smokers
Thirteen studies that presented the association between years of quitting smoking and risk of laryngeal cancer were defined and their summary estimate was 1.09 (95% CI 0.53–2.24), with significant heterogeneity between the studies (I 2 = 93.8%, p = 0.000). Sensitivity analysis via excluding every research was conducted to identify six related data of [9, 16, 18, 36] studies as the source of heterogeneity between study estimates. The subsequent analyses were limited to case–control studies (n = 7) involving 924 laryngeal cancer events and 2902 participants. The pooled estimate of the seven defined studies for the association between years of smoking cessation and risk of laryngeal cancer was 2.37 (95% CI 1.60–3.50; Table 2), with slight heterogeneity between the studies (I 2 = 22.9%, P = 0.255, fixed effects model adopted; Fig. 6).
Smoking cessation and risk of laryngeal cancer in former smokers in a meta-analysis (for definitions of the squares, lines, and diamond; see Fig. 1). Overall relative risk calculated with fixed effects model. ID1 0–15 years, ID2 ≥16 years, RR relative risk, CI confidence interval
The risk of laryngeal cancer kept elevated within the first 15 years of quitting smoking (RR 3.62, 95% CI 1.88–7.00) but declined in the 16 years and more after smoking cessation (RR 1.88, 95% CI 1.16–3.05, I 2 = 18.8%, p = 0.296, fixed effects model used). Sensitivity analysis by excluding every study revealed similar outcomes, suggesting the robustness of the outcomes. Subgroup analysis was not conducted to investigate source of study heterogeneity and the impact of latent confounding factors since this meta-analysis included under ten studies.
Influence of publication bias on the strength of the association
Publication bias was evaluated by visual inspection of a funnel plot, Egger’s test and Begg’s test (significant at p < 0.1; Figs. 7, 8). Evidence of publication bias was not detected for the association between current cigarette smoking (versus never smoking) and risk of laryngeal cancer (p = 0.225 with Begg’s test, p = 0.317 with Egger’s test; Table 2). Moreover, publication bias was not observed for studies presenting on the association between pack-years of cigarette smoking and risk of laryngeal cancer (p = 0.310 with Begg’s test, p = 0.110 with Egger’s test), the association between the number of cigarettes smoked per day and risk of laryngeal cancer(p = 0.566 with Begg’s test, p = 0.327 with Egger’s test), and the correlation between duration of cigarette smoking and risk of laryngeal cancer (p = 0.726 with Begg’s test, p = 0.844 with Egger’s test), respectively. Egger’s test and Begg’s test were not adopted to investigate publication bias for studies presenting on the association between years of quitting smoking and the risk of laryngeal cancer since this part of meta-analysis consisted of under ten studies.
Discussion
The present meta-analysis including all published eligible data identified a significant increase in the risk of laryngeal cancer in cigarette smokers compared with never smokers. The results of this meta-analysis including 14,292 laryngeal cancer events from 30 studies demonstrated strong association referring to dose–response and time–response between cigarette smoking and risk of laryngeal cancer for both men and women. The risks of laryngeal cancer were especially evident for individuals who smoked with 40 or more pack-years, subjects who smoked 30 or more cigarettes a day, and persons who smoked 40 or more years. The risks remained elevated for 15 years after smoking cessation but dropped afterwards. The vital public health implication was that the chance of developing laryngeal cancer might be distinctly decreased by quitting smoking, particularly among former cigarette smokers who had stopped smoking for 15 or more years.
The results of the meta-analysis pointing out the strong association between cigarette smoking and risk of laryngeal cancer supported the argument that cigarette smoking was more strongly associated with laryngeal cancer and the association was not influenced by the differences in sexes of subjects and controls [16, 30]. Comparing with the size of heterogeneity in geographical discrepancies or sample size, the differences in sexes were not the primary source of heterogeneity between study estimates. However, contrary to previous viewpoint that the great laryngeal cancer risk with smoking derived from the differential effects of cigarettes/day and not pack-years [27], this meta-analysis suggested that pack-years, frequency and duration of cigarette smoking were associated with a 4.6-fold, 5.8-fold, and 4.2-fold increased risk of laryngeal cancer, respectively. The pooled estimate of the 18 studies for the association between current smoking and risk of laryngeal cancer was 7.01 (95% CI 5.56–8.85), which was less than the result (RR 9.07; 95% CI 6.33–13.0) had reported [30]. The risks remained high for a 15 years after smoking cessation but dropped afterwards, compared with the previous view that the risk of upper aerodigestive tract cancer remained elevated in the first decade after smoking cessation but declined thereafter. The extent of the correlation was obviously greater among populations from Europe and America group compared with non-Europe and America group: the RRs were 7.89 (95% CI 5.77–10.77) and 6.64 (95% CI 4.83–9.12) separately, compared with the previous result that the difference corrected for the presence of publication bias between regions was reduced and became nonsignificant: the RR was 2.39 (95% CI 1.98–2.89) in Asian countries and 3.10 (95% CI 2.50–3.85) in non-Asian countries.
The main advantages of the present meta-analysis were based on a large number of studies and the first quantitative evaluation with respect to the association between laryngeal cancer risk and pack-years, frequency, duration and cessation of cigarette smoking. Cigarette smoking was regarded as the most vital risk factor for laryngeal cancer distinctly on the basis of the complete review of observational studies.
Sensitivity analysis was conducted by subgroup analysis and by excluding one study every time, investigating the effect of each study on the overall relative risk. The results of pooled relative risks and their 95% confidence intervals were changed after the first sensitivity analysis, while orientations of results were consistent. Most studies were of good quality with no evidence of selection bias, good outcome assessment of cohort studies, and with good comparability of the exposed and unexposed groups of case–control studies. Evidence of publication bias was not detected for the association between current cigarette smoking (versus never smoking) and risk of laryngeal cancer. Egger’s test and Begg’s test were not adopted to investigate publication bias for seven studies presenting on the association between years of quitting smoking and the risk of laryngeal cancer.
The current meta-analysis had several other strengths. Firstly, the study with large sample size of 14,292 laryngeal cancer patients enabled us to quantitatively evaluate the association between cigarette smoking and laryngeal cancer risk, proving to be more forceful than any single study. Secondly, the summary association between laryngeal cancer with different types of cigarette smoking, including pack-years, frequency, and duration, were comprehensively reviewed and surveyed from a new point of perspective. Moreover, the controlled potential confounding factors within the studies could be assessed with available data from recent published studies. Sensitivity analysis and subgroup analysis were conducted to look for sources of study heterogeneity and influence of potential confounders, respectively, through identifying differences in sample size, continent, design of researches, published years of researches, scores of studies by NOS standard, and genders of participants. Furthermore, the consistent direction of the overall outcomes from subgroup analysis showed robustness of our findings. The differences in genders of participants and controls did not affect the outcomes of effect estimates obviously. The present meta-analysis illustrated that the association between cigarette smoking and laryngeal cancer risk was weaker among non-Europe and America populations than association among Europe and America populations, which might attribute to differences in the components of cigarettes and smoking habits.
Findings from this study possessed vital public health implication. The prevention of laryngeal cancer incidence remained to be an important public health problem for studies. Millions of deaths from laryngeal cancer along with other smoking-related diseases could be avoided and the burden of laryngeal cancer worldwide would be decreased by banning cigarette on a population-wide scale.
The biological mechanism that cigarette smoking led to larynx carcinogenesis remained unclear. The cancers initiated by cigarette smoke could be attributed to its various elements containing nicotine, which is the major psychoactive component, and several other toxic constituents, such as nitrosamines, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, and polycyclic aromatic hydrocarbons. The major constituents of cigarette initiate a sequence of oncogenic events such as epigenetic changes, self-sufficiency in growth signals, evasion of apoptosis, continuous metastasis, and angiogenesis [29]. This research would give rise to new strategies for the treatment of laryngeal cancer which was caused by components of cigarettes. The capability to assess the impact of cigarette smoke on the condition of gene susceptibility with altered characters in carcinogenesis of laryngeal cancer was a promising domain for study and in favor of the better understanding of this important association. The deeper studies of correlation between risk of laryngeal cancer and similar risk factors such as tobacco chewing, smokeless tobacco, and other types of tobacco consumption and the carcinogenic effect of cigarette smoking on other subtypes of upper aerodigestive tract cancer required further investigation.
Potential limitations were present in this study. The majority of included studies were hospital-based or population-based case–control studies and thus prone to produce recall and selection bias. In addition, A few studies published in other languages for example in Polish had been searched and excluded since the limitation of inclusion criteria. Potential language bias generated when the studies not published in English or in Chinese were ruled out from the study. All available studies with disparate exposure definition were not comprised in subgroup analysis. Some studies could not eliminate former smokers from the reference group. The moderate heterogeneity within studies presenting about pack-years of cigarette smoking was mostly related to the dimension of the effect and might be interpreted by different design of studies and styles of cigarette smoking.
So far 13 eligible original studies that presented the correlation between years of quitting smoking and risk of laryngeal cancer were included. Sensitivity analysis via excluding every study was conducted to identify only seven studies for subsequent analyses. According to raw data in these seven studies, the years of quitting smoking were primarily categorized as within 15 and 16 years or more. The risk estimate in within 15 years group was generally and relatively greater than that in 16 years or more group. The characteristic of specifical risk-time development or the other points of time about the correlation between years of quitting smoking and risk of laryngeal cancer was not well understood, but certainly a critical question for future studies which would be based on lots of emerging original studies.
In summary, the results of meta-analysis presented powerful evidence that cigarette smoking was correlated with an approximately seven-time increase in risk of laryngeal cancer. The risk kept raised for 15 years after smoking cessation but dropped afterwards. This meta-analysis presented that pack-years, frequency and duration of cigarette smoking were associated with a 4.6-fold, 5.8-fold, and 4.2-fold increased risk of laryngeal cancer, respectively. The robust effect estimates were obtained through summarizing all the available studies, sensitivity analysis and subgroup analysis. The detailed analysis of association between laryngeal cancer risk and multiple risk factors such as occupational exposure, chewing of betel leaf, the other types of tobacco consumption, required future studies in more languages.
References
Ahrens W, Jockel KH, Patzak W, Elsner G (1991) Alcohol, smoking, and occupational factors in cancer of the larynx: a case–control study. Am J Ind Med 20(4):477–493
Applebaum KM, Furniss CS, Zeka A, Posner MR, Smith JF, Bryan J, Eisen EA, Peters ES, McClean MD, Kelsey KT (2007) Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst 99(23):1801–1810
Bosetti C, Gallus S, Franceschi S, Levi F, Bertuzzi M, Negri E, La Vecchia C (2002) Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. (Comparative Study Research Support, Non-U.S. Gov’t). Br J Cancer 87(5):516–518. doi:10.1038/sj.bjc.6600469
Bosetti C, Gallus S, Peto R, Negri E, Talamini R, Tavani A, La Vecchia C (2008) Tobacco smoking, smoking cessation, and cumulative risk of upper aerodigestive tract cancers (Multicenter Study Research Support, Non-U.S. Gov’t). Am J Epidemiol 167(4):468–473. doi:10.1093/aje/kwm318
Dai Q, Ji BT, Xu M, Gao YT (1994) Analysis of population attributable risk proportion (PARP) of some commonly seen cancer due to smoking in urban Shanghai. Tumor 14(4):208–211 (Chinese)
Dosemeci M, Gokmen I, Unsal M, Hayes RB, Blair A (1997) Tobacco, alcohol use, and risks of laryngeal and lung cancer by subsite and histologic type in Turkey. Cancer Causes Control 8(5):729–737
Durán de Alba LM, Roa Castro FM (2008) Risk factors for developing laryngeal cancer in adult population at the Hospital Español in Mexico City. Acta Otorrinolaringol Esp 59(8):367–370
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in metaanalysis detected by a simple, graphical test. BMJ 315(7109):629–634
Falk RT, Pickle LW, Brown LM, Mason MJ, Buffler PA, Fraumeni Jr JF (1989) Effect of smoking and alcohol consumption on laryngeal cancer risk in coastal Texas. Cancer Res 49:4024–4029
Feng J, Li L, Zhao YS, Tang SQ, Yang HB, Liu SX (2011) Interaction between CYP 2C19*3 polymorphism and smoking in relation to laryngeal carcinoma in the Chinese Han population (Research Support, Non-U.S. Gov’t). Genet Mol Res 10(4):3331–3337. doi:10.4238/2011.December.5.9
Feng S, Guo X, Wang Y, Li XT (2010). Risk factors of laryngeal carcinoma: a case–control analysis. J China Med Univ 39(6):474–475 (Chinese)
Franceschi S, Talamini R, Barra S, Baron AE, Negri E, Bidoli E, La Vecchia C (1990) Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in Northern Italy. Cancer Res 50(20):6502–6507
Freudenheim JL, Graham S, Byers TE, Marshall JR, Haughey BP, Swanson MK, Wilkinson G (1992) Diet, smoking, and alcohol in cancer of the larynx: a case–control study (Research Support, U.S. Gov’t, P.H.S.). Nutr Cancer 17(1):33–45. doi:10.1080/01635589209514171
Gallus S, Bosetti C, Franceschi S, Levi F, Negri E, Vecchia CL (2003) Laryngeal cancer in women: tobacco, alcohol, nutritional, and hormonal factors. Cancer Epidemiol Biomark Prev 12:514–517
Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P (2008) Tobacco smoking and cancer: a meta-analysis. Int J Cancer 122:155–164. doi:10.1002/ijc.23033
Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P (2008) Tobacco smoking and cancer: a meta-analysis (Meta-Analysis Research Support, Non-U.S. Gov’t). Int J Cancer 122(1):155–164. doi:10.1002/ijc.23033
Guo X (1993) A case–control study of the etiology of laryngeal cancer in Liaoning Province. Zhonghua er bi yan hou ke za zhi 28(4):219–221 (252)
Hashibe M, Boffetta P, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, Brennan P (2007) Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in Central Europe (Multicenter Study Research Support, Non-U.S. Gov’t). Am J Epidemiol 165(7):814–820. doi:10.1093/aje/kwk066
Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Boffetta P (2007) Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium (Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.). J Natl Cancer Inst 99(10):777–789. doi:10.1093/jnci/djk179
Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Boffetta P (2009) Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium (Multicenter Study Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.). Cancer Epidemiol Biomark Prev 18(2):541–550. doi:10.1158/1055-9965.EPI-08-0347
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Ishiguro S, Sasazuki S, Inoue M, Kurahashi N, Iwasaki M, Tsugane S (2009) Effect of alcohol consumption, cigarette smoking and flushing response on esophageal cancer risk: a population-based cohort study (JPHC study) (Research Support, Non-U.S. Gov’t). Cancer Lett 275(2):240–246. doi:10.1016/j.canlet.2008.10.020
Jayalekshmi PA, Nandakumar A, Akiba S, Gangadharan P, Koriyama C (2013) Associations of tobacco use and alcohol drinking with laryngeal and hypopharyngeal cancer risks among men in Karunagappally, Kerala, India—Karunagappally Cohort Study. PLoS One 8:8
Jee SH, Samet JM, Ohrr H, Kim JH, Kim S (2004) Smoking and cancer risk in Korean men and women. Cancer Causes Control 15:341–348
Lee KW, Kuo WR, Tsai SM, Wu DC, Wang WM, Fang FM, Ko YC (2005) Different impact from betel quid, alcohol and cigarette: risk factors for pharyngeal and laryngeal cancer (Research Support, Non-U.S. Gov’t). Int J Cancer 117(5):831–836. doi:10.1002/ijc.21237
Lu B, Li J, Gao Q, Yu W, Yang Q, Li X (2014) Laryngeal cancer risk and common single nucleotide polymorphisms in nucleotide excision repair pathway genes ERCC1, ERCC2, ERCC3, ERCC4, ERCC5 and XPA. Gene 542(1):64–68
Lubin JH, Purdue M, Kelsey K, Zhang ZF, Winn D, Wei Q, Hayes RB (2009) Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case–control studies (Meta-Analysis Research Support, N.I.H., Intramural). Am J Epidemiol 170(8):937–947. doi:10.1093/aje/kwp222
Maasland DH, van den Brandt PA, Kremer B, Goldbohm RA, Schouten LJ (2014) Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: results from the Netherlands Cohort Study (Research Support, Non-U.S. Gov’t). BMC Cancer 14:187. doi:10.1186/1471-2407-14-187
Maurya SS, Katiyar T, Dhawan A, Singh S, Jain SK, Pant MC, Parmar D (2015) Gene–environment interactions in determining differences in genetic susceptibility to cancer in subsites of the head and neck. Environ Mol Mutagen 56(3):313–321
Moghaddam AA, Huxley RR, Lam TH, Woodward M (2009) Risk of upper aerodigestive tract cancer associated with smoking with and without concurrent alcohol consumption. Mt Sinai J Med 76:392–403. doi:10.1002/msj.2012510.1002/MSJ
Moura MA, Bergmann A, Aguiar SS, Thuler LC (2014) The magnitude of the association between smoking and the risk of developing cancer in Brazil: a multicenter study. BMJ Open 4(2):e003736. doi:10.1136/bmjopen-2013-003736
Norman RP (2002) Risk factors for oesophageal, lung, oral and laryngeal cancers in black South Africans. Br J Cancer 86:1751–1756
Ramroth H, Dietz A, Becher H (2011) Intensity and inhalation of smoking in the aetiology of laryngeal cancer. Int J Environ Res Public Health 8(4):976–984
Risch A, Ramroth H, Raedts V, Rajaee-Behbahani N, Schmezer P, Bartsch H, Dietz A (2003) Laryngeal cancer risk in Caucasians is associated with alcohol and tobacco consumption but not modified by genetic polymorphisms in class I alcohol dehydrogenases ADH1B and ADH1C, and glutathione-S-transferases GSTM1 and GSTT1 (Research Support, Non-U.S. Gov’t). Pharmacogenetics 13(4):225–230. doi:10.1097/01.fpc.0000054081.64000.b8
Sterne JA, Egger M, Smith GD (2001) Investigating and dealing with publication and other biases in metaanalysis. BMJ 323(7304):101–105
Talamini R, Bosetti C, La Vecchia C, Dal Maso L, Levi F, Bidoli E, Franceschi S (2002) Combined effect of tobacco and alcohol on laryngeal cancer risk: a case–control study. Cancer Causes Control 13(10):957–964
Tavani A, Negri E, Franceschi S, Barbone F, La Vecchia C (1994) Attributable risk for laryngeal cancer in Northern Italy. Cancer Epidemiol Biomark Prev 3(2):121–125
Toporcov TN, Znaor A, Zhang ZF, Yu GP, Winn DM, Wei Q, Filho VW (2015) Risk factors for head and neck cancer in young adults: a pooled analysis in the INHANCE consortium. Int J Epidemiol 44(1):169–185
Vaezi MF, Qadeer MA, Lopez R, Colabianchi N (2006) Laryngeal cancer and gastroesophageal reflux disease: a case–control study. Am J Med 119(9):768–776. doi:10.1016/j.amjmed.2006.01.019
Yun YH, Jung KW, Bae JM, Lee JS, Shin SA, Min Park S, Yul Huh B (2005) Cigarette smoking and cancer incidence risk in adult men: national Health Insurance Corporation Study. Cancer Detect Prev 29(1):15–24. doi:10.1016/j.cdp.2004.08.006
Zeka A, Gore R, Kriebel D (2003) Effects of alcohol and tobacco on aerodigestive cancer risks: a meta-regression analysis. Cancer Causes Control 14:897–906
Zhou J, Liu F, Zhang D, Chen B, Li Q, Zhou L, Tao L (2014) Significance of MDM2-309 polymorphisms and induced corresponding plasma MDM2 levels in susceptibility to laryngeal squamous cell carcinoma. DNA Cell Biol 33(2):88–94
Zvrko E, Gledović Z, Ljaljević A (2008) Risk factors for laryngeal cancer in Montenegro. Arh Hig Rada Toksikol 59(1):11–18
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Jing-Jing Zuo declares that he has no conflict of interest. Ze-Zhang Tao declares that he has no conflict of interest. Chen Chen declares that he has no conflict of interest. Zhang-Wei Hu declares that he has no conflict of interest. Ye-Xing Xu declares that she has no conflict of interest. An-Yuan Zheng declares that he has no conflict of interest. Yi Guo declares that he has no conflict of interest.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81372880), and the Natural Science Foundation of Hubei Province of China (No. 2012FFA045).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zuo, JJ., Tao, ZZ., Chen, C. et al. Characteristics of cigarette smoking without alcohol consumption and laryngeal cancer: overall and time-risk relation. A meta-analysis of observational studies. Eur Arch Otorhinolaryngol 274, 1617–1631 (2017). https://doi.org/10.1007/s00405-016-4390-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4390-x