Abstract
Voice symptoms are frequently reported early after thyroidectomy, even in the absence of laryngeal nerves injury. We evaluated the short-term outcomes of these functional alterations. Thirty-nine patients were enrolled in a prospective observational trial, evaluating voice function before and 3 months after uncomplicated thyroidectomy, using VoiSS as assessed using a validated patient rated questionnaire; and perceptual voice analysis using GRBAS scale (Grade, Roughness, Breathiness, Asthenia, Strain). Impact of dysphonia on patient’s life using VoiSS questionnaire revealed differences between pre- and postoperative assessment. There was statistically significant worsening in the impairment subgroup of VoiSS (p = 0.027). GRBAS evaluation was consistent between the three independent raters but showed differences between pre- and postoperative voice assessment. Age, TSH and a preoperative finding of laryngopharyngeal reflux significantly predicted quality of voice after thyroid surgery (all p < 0.004), as identified by the GRBAS assessment tool, but not type of surgery, gender or smoking status; although prediction of total variance in changes of voice was modest (r 2 = 0.07). Voice changes may occur after thyroidectomy without evident laryngeal nerve injury. Patients should be made aware of possible mild changes in voice even after uncomplicated thyroid surgery and this might be considered to be part of the informed consent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Thyroid surgery rates have tripled over the past three decades [1]. One of the major complications of the thyroid surgery is an injury of recurrent laryngeal nerve which can cause permanent damage of the patient’s voice. The symptoms affecting voice are vocal fatigue, reduced voice range, effortful speech and breathy voice. Voice symptoms are frequently reported early after thyroidectomy even in the absence of laryngeal nerves injury. Voice problems after uncomplicated thyroidectomy can be caused by other factors such as disturbance of extralaryngeal skeleton [2], with changes of acoustic parameters being reported to be different between male and female patients [3]. These deficits can persist permanently, although not all acoustic changes are deemed to be clinically relevant [3].
Various tools for the assessment of the quality of the voice in post-thyroidectomy setting have been reported, which include Acoustic Voice Analysis, Voice Handicap Index, Dysphonia Severity Index, Maximum Phonation Time or Fundamental Frequency [4–7].
The majority of previous evidence reported short-term voice changes after thyroidectomy, assessing changes by various subjective and objective methods [2, 4, 5, 8, 9]. Lombardi and colleagues [8] followed 39 patients after total thyroidectomy and obtained Voice Impairment Scores (VIS), which is a tool used for subjective evaluation of the voice. The mean postoperative VIS was significantly higher than the preoperative VIS at 1 week and 1 month after TT (13.7 (±10.7) and 9.6 (±8.9) vs 4.4 (±7.0), respectively; p < 0.05), but not 3 months after TT (6.7) [8]. In another report, Lombardi used acoustic voice analysis and maximum phonation time, 3 and >12 months postoperatively in 110 patients [9]. The percentage of patients with symptoms 1 week after surgery was significantly higher than preoperatively, but significantly decreased at long-term evaluation [9].
Henry and colleagues evaluated 62 patients after thyroidectomy and reported that Dysphonia Severity Index (DSI) changes from baseline to 1–4 weeks were highly predictive of the negative voice outcome 6 months after surgery [4]. DSI is a tool designed to establish an objective and quantitative correlate of the perceived vocal quality. Stojadinovic and colleagues used patient self-assessment of voice handicap; authors concluded that Voice Handicap Index reliably identifies voice dysfunction after thyroidectomy. Patients with a change in VHI ≥25 from preoperative baseline warrant early referral to speech pathology and laryngology [5]. Observational comparative studies by de Pedro Netto and colleagues in 100 thyroidectomy patients and 30 matched controls who underwent the breast surgery evaluated the Voice handicap index and reported that voice complaints were more frequently registered in the thyroid group rather than in the control group [10]. Authors concluded that orotracheal intubation was just one of the multiple factors involved and that there are mild voice changes to be expected even in uncomplicated thyroidectomy [10]. Sinagra and colleagues measured the cycle-to-cycle variations of amplitude (Shimmer) and fundamental frequency (Fo) in 46 consecutive patients who underwent total thyroidectomy [11]. Voice fatigue during phonation was the most common symptom after thyroidectomy. Forty patients (87 %) stated that their voices had changed since the operation, and common complaints were voice alteration while speaking loudly, changes in voice pitch, and voice disorder while singing. Changes in the Fo and Shimmer values in smokers versus nonsmokers were not significantly different [11].
In our work, we used the short-term outcomes of functional voice alterations using VoiSS questionnaires and clinician-based voice assessment protocol GRBAS, based on their universal availability and the goal to focus on patient’s and therapist’s perspectives [6, 12, 13]. The VoiSS questionnaire and GRBAS evaluation scores had not been investigated in this context previously.
Patients and methods
This was a prospective observational clinical trial, evaluating voice function before and 3 months after uncomplicated thyroidectomy, using laryngeal examination via videolaryngoscopy. Research was limited to secondary use of information previously collected in the course of normal care and data were anonymized before the conduction of statistical analyses. Therefore, this research did not fulfill the requirements for Research Ethics Committee (REC) review, in accordance with the Governance Arrangements for Research Ethics Committees (GAfREC), revised by the UK Health Department in February 2012 [14]. All clinical investigations were conducted in accordance with the guidelines in the Declaration of Helsinki.
Thirty-nine patients were enrolled in a prospective observational trial, evaluating voice function before and 3 months after uncomplicated thyroidectomy, using laryngeal examination via videolaryngoscopy. Inclusion criteria were adult patients undergoing hemithyroidectomy or total thyroidectomy with normal voice, without previous surgical intervention on the neck (apart from completion thyroidectomy) or in the larynx. We excluded persons under 18 years of age, patients unable or unwilling to give informed consent and patients with known thyroid cancer. We also excluded patients who have abnormal laryngeal movement at any stage during the study duration. Thyroidectomy was performed for clinical indications that included thyroid confined malignancy, benign nodules or cysts, suspicious findings on fine needle aspiration biopsy, dysphagia from cervical esophageal compression, or dyspnea from airway compression. Other indications for thyroidectomy included multinodular goiter, Hashimoto’s and other types of thyroiditis, and thyromegaly with significant cosmetic compromise.
At the preoperative visit, laryngeal endoscopy was performed and age, gender, smoking status, extent of surgery, thyroid function status and the presence or absence of laryngopharyngeal reflux (LPR) were recorded. A diagnosis of LPR was based on combination of typical symptoms such as tickling or burning sensations in the throat, frequent throat clearing, chronic cough, hoarseness, post-nasal drip; and laryngeal findings, i.e., red, irritated, and swollen voice box. Reflux Symptom Index (RSI) was calculated in each patient and a score higher than 13 was considered to be abnormal [15].
Thyroidectoctomy was performed as hemithyroidectomy, one-step total thyroidectomy, or completion thyroidectomy; as clinically appropriate. The operative procedures were performed by one of two participating ENT consultants and were comparable for all patients. In each case the recurrent laryngeal nerve was identified and preserved. The superior laryngeal nerve was identified in 31 cases. In the remaining eight cases the superior laryngeal nerve was sought but not identified (Fig. 1). The individual vessels were ligated and divided individually to avoid damage to the nerve.
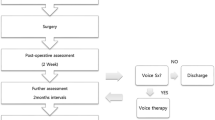
Patients were seen on three occasions: preoperatively; 2 weeks and 3 months after surgery. The larynx was evaluated on all three visits using flexible nasolaryngoscope. Voice was evaluated at the preoperative visit and 3 months postoperatively. All 39 patients had normal laryngeal movement on laryngoscopy at all recorded visits. For assessment, we used videolaryngoscope inserted through the nasal cavity, visualized the larynx and asked patient to perform sustained phonation at different pitches and asked them to count from zero to ten).
VoiSS questionnaire has been used to evaluate the impact that dysphonia has in patient‘s life [12]. VoiSS is a self-report measure quantifying patient’s assessment of the impact of the voice disorder. The tool consists of a 30-item questionnaire and comprises three factors—impairment (15 items), emotional (8 items) and related physical symptoms (7 items). The VoiSS is psychometrically the most robust and extensively validated self-report voice measure available [6].
Voice recording of the standard text (the rainbow passage) was made at the preoperative visit and 3 months postoperatively. Clinician-based voice assessment protocol GRBAS (grade, roughness, breathiness, asthenia, strain) was evaluated by three independent experienced blinded raters. All three judges had extensive experience and had been working as senior speech and language therapists specializing in voice for more than 10 years.
Statistical analyses
Data are presented as mean ± SE. Kolmogorov–Smirnoff test was used to investigate whether data were normally distributed. Not normally distributed data and ordinal data were compared using Wilcoxon signed-rank tests, or Kruskal–Wallis test for k independent samples, as appropriate. Bootstrapped linear regression models (set as 1000 bootstrap samples) were used to evaluate the impact of variables on voice changes, with pre- and postoperative voice assessment as the respective dependent variables and gender, age, smoking status, LPR, TSH value and type of surgery as independent variables. Reliability analyses were expressed using Cronbach’s alpha, with a value >0.70 assumed to be acceptable. A p value <0.05 was assumed to be statistically significant. Analyses were performed using SPSS version 22 (SPPS, Chicago, IL, USA).
Results
Impact of dysphonia on patient’s life using VoiSS questionnaires showed significant differences for voice impairment between the respective pre- and postoperative assessments (p < 0.001). Other parameters did not significantly change. Therefore, the observed change in total VoiSS assessment (p = 0.001) was mainly driven by the significant change in the “voice impairment” parameter (Table 1).
GRBAS evaluation pre- and 3 months post operatively as assessed using reliability analyses was consistent between the three independent assessors (Cronbach’s alpha 0.917). Subtest analyses revealed significant differences pre- and post operatively in roughness and strain part of assessment, and a strong trend in grade (Table 2).
Preoperative predictors for deterioration in the quality of voice total score identified age (95 % CI −0.267, −0.054, p = 0.004), LPR (95 % CI −12.662, −2.842, p = 0.003) and TSH (95 % CI −2.473, −0.686, p = 0.001) as significant predictive factors, but only 7 % (adjusted R 2 0.073) of the variance in voice quality was explained when including all factors (age, gender, smoking status, TSH, type of surgery and LPR) in the model. Postoperatively, the effects of age (p = 0.37), LPR (p = 0.18) and TSH (p = 0.69) disappeared, but instead gender (95 % CI −11.532, −2.846, p = 0.001), type of surgery (95 % CI 0.696, 8.699, p = 0.014), and smoking status (95 % CI −4.901, −0.205, p = 0.03) became significant predictors (95 % CI 0.268, 9.937, p = 0.039), with remaining variables not significantly contributing to explain the variance in voice quality. Again, only a small proportion of the variance in voice quality was explained by this model (adjusted R 2 0.094).
Discussion
Voice disturbances after thyroidectomy have been traditionally attributed to direct injury to laryngeal nerves, resulting in vocal cord dysfunction. Nevertheless, it is well known among thyroid surgeons that after thyroidectomy, most patients complain of some degree of mainly transient voice changes, even in the absence of laryngeal nerve injuries—postthyroidectomy syndrome.
Voice changes after uncomplicated thyroidectomy have been assessed previously [5, 8, 10, 16]. In the past, voice outcomes have relied upon perceptual judgement of voice quality and acoustic measurement of a periodicity in the speech signal. It became apparent that the severity of the voice disorder may not reflect the impact that the dysphonia has in the patient’s life [12].
Most of these published work reported changes in the early post-thyroidectomy period, e.g., 1 week after surgery. Some evidence [8, 9] showed normal voice in the period 3 months after the surgery. In this respect, our data show negative voice outcomes 3 months after the surgery in terms of impact of dysphonia on patient’s life—voice impairment parameter of VoiSS.
We have identified only one study prospectively recruiting 44 patients, which did not show an impact on voice after thyroid surgery. Subjective (auditory perceptual evaluation and videolaryngostroboscopy) and objective (aerodynamic, vocal range, acoustic, and Dysphonia Severity Index measurements) assessment techniques were used using the Dysphonia Severity Index. No permanent change of the vocal performance had been [16]. This is in contrast with other studies [2] including our study (where subjective methods for voice evaluation only were used) and may relate to both the specific characteristics of the investigated cohort of van Lierde and colleagues and different techniques used; further research using both subjective and objective assessment methods are needed to confirm the findings in independent cohorts.
Recent published work suggested implementing several measures to improve voice outcomes after the surgery. A single preoperative dose of dexamethasone may decrease voice changes after thyroidectomy [17]. The AAO Clinical Practice Guideline: Improving Voice Outcomes after Thyroid Surgery published in 2013 recommends to implement below mentioned steps to the standard care [1]; and the group agreed that voice outcomes could potentially be improved preoperatively, intraoperatively and postoperatively. Steps should include preoperative laryngoscopy and voice assessment, preserve the external branch of the superior laryngeal nerve, document whether there has been a change in voice between 2 weeks and 2 months following thyroid surgery, refer a patient to an otolaryngologist when abnormal vocal fold mobility is identified after thyroid surgery; or counsel patients with voice change or abnormal vocal fold mobility after thyroid surgery on options for voice rehabilitation [1].
Voice changes may occur after thyroidectomy without evident superior and recurrent laryngeal nerve injury. In our study, we identified a significant influence of LPR predicting negative outcomes on the quality of voice after thyroid surgery. It needs to be investigated in future studies whether preoperative treatment of LPR may beneficially influence post-thyroidectomy voice quality.
Our data are had been collected prospectively and were covering various types of thyroid surgeries with no difference between surgical methods. The low number of assessors is a limitation of this study but reliability analyses indicated consistency between the three independent assessors. The GRBAS tool showed to be robust for assessment which was consistent within three independent raters. In our continuing work, we are focusing on long-term evaluation of voice outcomes after the procedure.
Conclusions
Findings of the present study suggest that GRBAS assessment and the VoiSS questionnaire could be valuable tools in assessing the quality of voice in thyroidectomy setup. Patients’ complaints regarding the voice impairment after thyroid surgery without laryngeal nerve injury were non-specific and include mostly slightly rough voice and vocal fatigue. Our results indicate presence of short-term (3 months post operatively) voice changes after thyroid surgery without laryngeal nerve damage. Patients should be made aware of possibility of mild changes in voice after uncomplicated thyroid surgery and this might be considered to be part of the informed consent.
References
Chandrasekhar SS, Randolph GW, Seidman MD, Rosenfeld RM, Angelos P, Barkmeier-Kraemer J, Benninger MS, Blumin JH, Dennis G, Hanks J, Haymart MR, Kloos RT, Seals B, Schreibstein JM, Thomas MA, Waddington C, Warren B, Robertson PJ, American Academy of O-H, Neck S (2013) Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg 148(6 Suppl):S1–37. doi:10.1177/0194599813487301
Hong KH, Kim YK (1997) Phonatory characteristics of patients undergoing thyroidectomy without laryngeal nerve injury. Otolaryngol Head Neck Surg 117(4):399–404
Minni A, Ruoppolo G, Barbaro M, Di Lorenzo E, Sementilli G, Bononi M (2014) Long-term (12 to 18 months) functional voice assessment to detect voice alterations after thyroidectomy. Eur Rev Med Pharmacol Sci 18(12):1704–1708
Henry LR, Helou LB, Solomon NP, Howard RS, Gurevich-Uvena J, Coppit G, Stojadinovic A (2010) Functional voice outcomes after thyroidectomy: an assessment of the Dsyphonia Severity Index (DSI) after thyroidectomy. Surgery 147(6):861–870. doi:10.1016/j.surg.2009.11.017
Stojadinovic A, Henry LR, Howard RS, Gurevich-Uvena J, Makashay MJ, Coppit GL, Shriver CD, Solomon NP (2008) Prospective trial of voice outcomes after thyroidectomy: evaluation of patient-reported and clinician-determined voice assessments in identifying postthyroidectomy dysphonia. Surgery 143(6):732–742. doi:10.1016/j.surg.2007.12.004
Wilson JA, Webb A, Carding PN, Steen IN, MacKenzie K, Deary IJ (2004) The Voice Symptom Scale (VoiSS) and the Vocal Handicap Index (VHI): a comparison of structure and content. Clin Otolaryngol Allied Sci 29(2):169–174. doi:10.1111/j.0307-7772.2004.00775.x
Akyildiz S, Ogut F, Akyildiz M, Engin EZ (2008) A multivariate analysis of objective voice changes after thyroidectomy without laryngeal nerve injury. Arch Otolaryngol Head Neck Surg 134(6):596–602. doi:10.1001/archotol.134.6.596
Lombardi CP, Raffaelli M, D’Alatri L, Marchese MR, Rigante M, Paludetti G, Bellantone R (2006) Voice and swallowing changes after thyroidectomy in patients without inferior laryngeal nerve injuries. Surgery 140(6):1026–1032. doi:10.1016/j.surg.2006.08.008 (discussion 1032–1024)
Lombardi CP, Raffaelli M, De Crea C, D’Alatri L, Maccora D, Marchese MR, Paludetti G, Bellantone R (2009) Long-term outcome of functional post-thyroidectomy voice and swallowing symptoms. Surgery 146(6):1174–1181. doi:10.1016/j.surg.2009.09.010
de Pedro Netto I, Fae A, Vartanian JG, Barros AP, Correia LM, Toledo RN, Testa JR, Nishimoto IN, Kowalski LP, Carrara-de Angelis E (2006) Voice and vocal self-assessment after thyroidectomy. Head Neck 28(12):1106–1114. doi:10.1002/hed.20480
Sinagra DL, Montesinos MR, Tacchi VA, Moreno JC, Falco JE, Mezzadri NA, Debonis DL, Curutchet HP (2004) Voice changes after thyroidectomy without recurrent laryngeal nerve injury. J Am Coll Surg 199(4):556–560. doi:10.1016/j.jamcollsurg.2004.06.020
Deary IJ, Wilson JA, Carding PN, MacKenzie K (2003) VoiSS: a patient-derived Voice Symptom Scale. J Psychosom Res 54(5):483–489
Mehanna R, Hennessy A, Mannion S, O’Leary G, Sheahan P (2015) Effect of endotracheal tube size on vocal outcomes after thyroidectomy: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg 141(8):690–695. doi:10.1001/jamaoto.2015.1198
Department UH (2012) Does-my-project-require-rec- review. http://www.hra.nhs.uk/documents/2013/09/does-my-project-require-rec-reviewpdf. Accessed 17 May 2016
Belafsky PC, Postma GN, Koufman JA (2002) Validity and reliability of the reflux symptom index (RSI). J Voice 16(2):274–277
Van Lierde K, D’Haeseleer E, Wuyts FL, Baudonck N, Bernaert L, Vermeersch H (2010) Impact of thyroidectomy without laryngeal nerve injury on vocal quality characteristics: an objective multiparameter approach. Laryngoscope 120(2):338–345. doi:10.1002/lary.20762
Nasiri S, Shafag S, Khorgami Z, Sodagari N, Aminian A, Hedayat A (2013) Does corticosteroid have any beneficial effect on voice change after thyroidectomy? Am Surg 79(12):1258–1262
Acknowledgments
Hisham Mehanna for methodological guidance; June Jones and Gemma M. Jones for support with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Tedla, M., Chakrabarti, S., Suchankova, M. et al. Voice outcomes after thyroidectomy without superior and recurrent laryngeal nerve injury: VoiSS questionnaire and GRBAS tool assessment. Eur Arch Otorhinolaryngol 273, 4543–4547 (2016). https://doi.org/10.1007/s00405-016-4163-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4163-6