Abstract
Epidemiologic and clinicopathologic features, therapeutic strategies, and prognosis for acinic cell carcinoma of the major and minor salivary glands are critically reviewed. We explore histopathologic, histochemical, electron microscopic and immunohistochemical aspects and discuss histologic grading, histogenesis, animal models, and genetic events. In the context of possible diagnostic difficulties, the relationship to mammary analog secretory carcinoma is probed and a classification is suggested. Areas of controversy or uncertainty, which may benefit from further investigations, are also highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The International Head and Neck Scientific Group regarded that a series of articles revisiting the major epithelial salivary malignancies in the light of contemporary knowledge would be of interest. In this respect, an article on adenoid cystic carcinoma [1] as well as on mucoepidermoid carcinoma has recently been published [2] and is now followed by the present article on acinic cell carcinoma (AcCC). This was deemed appropriate as both mucoepidermoid carcinoma and AcCC are characterized by innate acinar differentiation and an often favorable prognosis [2, 3], features interesting enough to result in intensive study and accumulating literature. The structure of the present article and the principles of our approach are similar to those of the previous [1, 2]. We review salivary gland AcCC, critically appraising the recent literature and integrating recent findings into the existing knowledge base, predicated on extensive clinical experience, epidemiological, clinicopathological, imaging and genomic aspects that determine our management decisions and consequent prognosis.
Definition and brief historical survey
The World Health Organization (WHO) currently defines salivary AcCC as “a malignant epithelial neoplasm of salivary glands in which at least some of the neoplastic cells demonstrate serous acinar cell differentiation, which is characterized by cytoplasmic zymogen secretory granules. Salivary ductal cells are also a component of this neoplasm” [3]. Commitment to the characterisation of the secretory granules as zymogen and interpretation of the cells lacking obvious secretory granules as ductal cells may be criticized. The definition is, however, useful because it emphasizes the presence of a structural component other than serous-like acinar cells.
Godwin et al. [4] traced the earliest cases back to the 1890s, Nasse [5] being generally regarded as having described the first case in 1892 as a ‘blue dot tumor’, because of the appearance of what we now know are intracytoplasmic zymogen granules. It is likely that the serous cell phenotypes and apparent circumscription of the tumor accounted for the description of serous cell or acinar adenomas in the earlier literature [6]. Buxton et al. [7] probably described the first cases of AcCC with a straightforward malignant behavior. Foote and Frazell [8] are usually credited with the “modern” morphological descriptions of the tumor, but it was oral pathologists who subsequently took the lead. Their efforts culminated in 1965 when a group led by Abrams published a detailed clinicopathologic study of 77 cases of AcCC of major salivary glands from the archives of the Armed Forces Institute of Pathology (AFIP), which defined particular growth patterns and tumor-cell types [9]. In the 1970s, the publication of the World Health Organization (WHO) histological classification of salivary gland tumors [10] and also the seminal volume by Thackray and Lucas [11] spearheaded the now discarded term “acinic cell tumor” (with the suggestion to only use the term “carcinoma” if the tumor “happens to metastasize”) and general pathologists with a special interest in head and neck entered the field [12–14]. Oral pathologists responded by defining clinicopathologic features of AcCC in minor salivary glands [15, 16], revisiting the AFIP archives of 294 cases [17] and aptly presenting the experience in the AFIP atlas [18]. The accumulating clinicopathologic experience together with investigative approaches using modalities such as electron microscopy, histochemistry, and immunohistochemistry substantially increased our understanding of AcCC. There seemed little wishing for and AcCC did not feature in reviews of advances in salivary pathology [19, 20]. However, the notion of AcCCs entirely composed of non-descript cells lacking secretory granules, illustrative per se of the inherent difficulties in precisely characterizing cells of simple phenotypes, should warrant a certain apprehension. This proved well founded in 2010 when the so-called mammary analog secretory carcinoma (MASC), a low-grade salivary malignancy that is histologically similar to AcCC of non-serous acinar cells and harbors the ETV6–NTRK3 translocation, was reported [21]. Subsequently Bishop et al. [22] re-classified most non-parotid AcCC of non-serous cells as MASCs. Whether MASC is a distinct entity remains to be established, which is discussed below (see “Proposed classification”), but is a concept that should be considered when the earlier literature is reviewed.
Whereas most clinicians still associate AcCC to a good prognosis, recent studies increase our awareness of the propensity of this tumor for lymphatic invasion and distant metastases, developing in a protracted and unpredictable clinical course. Indeed, (late) distant metastases to the lungs, pleura, brain, peritoneum, paraaortic, paratracheal, and mediastinal lymph nodes, as well as cutaneous metastases, have been described, especially in the de-differentiated subgroup of AcCC, nowadays commonly referred to as “acinic cell carcinoma with high-grade transformation” [13, 23–25].
Epidemiology
Unfortunately, the highly desirable population-based studies provide little information beyond incidence and survival [26–28]. Institution-based studies are more detailed, but report limited number (up to 35) of cases collected over a long period and treated without standardized protocols, which makes statistical evaluation difficult [29–32].
In western countries, salivary gland carcinomas (SGCs) account for about 4 % of all head and neck cancers, approximately 80 % occurring in the parotid [33]. About one out of six parotid cancers is AcCC [34], which is supported by a nationwide study in Netherlands, where 15 % of parotid malignancies were AcCC [26]. A recent surveillance, epidemiology, and end results (SEER) analysis from 1973 to 2009 indicated that AcCC comprises 11 % of all salivary gland malignancies, with an average annual incidence of 0.13 cases per 100,000 patients per year during the 36 years the study encompassed [28]. Incidence trend analysis, stratified for gender and race, indicated a significant annual increase (annual percentage change of 1.06 %) [28]. Rather than being genuine, this trend is attributable to improved and increasingly widely known histopathologic diagnostic criteria. The SEER analysis also indicated a higher average annual incidence for females (0.15 cases per 100,000 patients) in comparison to males (0.11 cases per 100,000 patients). This correlates with a consistent slight female predominance in institutional [35, 36] and population-based series. The latter report female:male incidence ratios ranging from 1.43:1 to 1.57:1 [27, 28, 37].
The age distribution of AcCC seems quite even throughout all decades, with one-third of patients below the age of 40, one-third between 40 and 59, and one-third above 60 years [28]. This corresponds to the findings in a series from the MD Anderson Cancer Center (MDACC) [36]. With a median age at diagnosis of 52 years, AcCC tends to occur at a younger age than other SGCs [27]. Children are very rarely affected by SGCs, but when they are, the most frequently observed histologic type is mucoepidermoid carcinoma, followed by AcCC [38, 39].
AcCC is predominantly diagnosed in whites (85 %) and less frequently in blacks (7 %) or other racial backgrounds (8 %) [28, 32]. Very little is known regarding risk factors for AcCC. Although familial predisposition and previous radiation exposure have been considered, no cases were noted among long-term atomic bomb survivors [40] and descriptions of familial occurrence are very sparse [41]. One case has been reported in an individual with Cowden syndrome [42], and there is a recent case report of AcCC of the breast developing in a BRAC1 mutation carrier [43].
Clinical features
In the major salivary glands, the parotid is most commonly affected, the typical clinical presentation being a slow-growing swelling. Symptoms are often lacking, which often results in late diagnosis. Pain or fixation to surrounding tissues herald poor prognosis [35]. Nodal metastasis is extremely uncommon at presentation. In the MDACC series mentioned above, only 12 of 155 patients (8 %) had nodal disease, even when 75 % presented with persistent or recurrent AcCC [36]. In another series from the Memorial Sloan Kettering Cancer Center (MSKCC), three out of 35 patients (9 %) presented with nodal disease; also uncommon in this series were pain (n = 5; 13 %) and cranial nerve VII dysfunction (n = 1; 3 %) [32].
AcCC is far less common in minor salivary glands. A population-based report identified 736 cases of parotid AcCC (91 %) compared to only 42 cases (5 %) in other major and 35 cases (4 %) in minor salivary glands [27]. In addition, AcCC of minor salivary glands accounts for about 9 % in a SEER database [44]. The trend is also a feature of institutional series [36] and in case reports [45, 46]. Whether this relates to a generally decreased proportion of serous acinar cells in normal minor salivary glands, is unknown. In contrast to other types of minor SGCs, which mainly occur in the palate, [47] AcCC mainly occurs in the buccal mucosa and upper lip [31]. A small minority of AcCC arises in the sinonasal area [44, 48] or the larynx [49], but these are outside the scope of the present article.
Bilateral AcCC is highly controversial. While some urge caution or are unconvinced, [50] others suggest that AcCC is the most frequently reported bilateral SGC [51, 52]. For completeness, non-salivary AcCC have been described in the lacrimal gland, pancreas, and breast. The tumors in the pancreas are referred to as acinar cell carcinoma [43, 53].
Pre-operative assessment
Surgery is the first and most important step in the management, if technically feasible and if there are no medical contraindications. Pre-operative assessment of AcCC is similar to that of other tumors of the major salivary glands and involves imaging and needling procedures. Since AcCC often presents as a swelling, with little to suggest malignancy, pre-operative assessment aims at assessing localization, extent, indicators of malignancy, as in the case of parotid AcCC, these factors will determine the risk to the facial nerve during surgery [54]. Imaging can be omitted without detriment to further management in mobile, circumscribed tumors where localization and extent are clinically obvious. It is strongly recommended when a glandular swelling is associated with impaired mobility or when involvement of deeper structures/cranial nerves is suspected [55–58]. For AcCC, impaired mobility is typically seen in larger tumors or, more frequently, in local recurrences.
Pending on particular circumstances, pre-operative imaging includes ultrasound (US) (Fig. 1), computed tomography (CT), magnetic resonance imaging (MRI) (Fig. 2), and positron emission tomography (PET) [54].
A recent study comparing US and CT indicated that most primary AcCCs show ‘benign’ imaging features reconcilable with the often favorable prognosis of the tumor. On US, AcCC appeared lobular, rather defined, hypoechoic, heterogeneous and poorly vascularized (Fig. 1); on CT, it appeared regular and variably defined with limited heterogeneous enhancement [59]. The study supported the earlier findings of Suh et al. [60], who described CT qualities of AcCC in relation to histopathologic features.
MRI is superior to CT in assessing parotid, stylomastoid foramen and any facial nerve invasion/perineural extension (Fig. 2) [56, 57, 61, 62]. It is particularly indicated for patients with recurrent or residual AcCC and favored in tertiary centers where these patients are usually referred. As an example, 75 % of patients treated in MDACC had residual or recurrent disease; for these patients a meaningful statement on the feasibility of further treatment without an adequate was judged impossible without MRI [36].
PET with or without CT should be considered in clinically/radiologically suspected or needling-procedure proven, advanced stage, salivary malignancies, to exclude gross, distant disease. This is a very uncommon situation in AcCC [54]. PET may be, however, recommended post-operatively when a histologic diagnosis of AcCC with high-grade transformation is established [63–65].
The needling procedures are usually ultrasound guided and include fine needle aspiration cytology (FNAC) and core biopsy (USCB). FNAC is an integral part of the pre-operative assessment, and it has been repeatedly emphasized that the value of FNAC reflects the experience of the operator, yield of material and expertise of the pathologist interpreting the aspirate, which in turn are influenced by the number of salivary tumors managed in particular institutions. Data on the role of FNAC in diagnosing AcCC (Fig. 3a) are limited. Overall sensitivity is low, often due to a false negative interpretation of tumoral acinar structures (see “Pathology”) as normal parenchyma. Accuracy of 17 % (two out of 12) [30] and a specificity of 27 % (four out of 15) [66] have been reported. Cystic tumors (see “Pathology” section) present additional problems, since obtained aspirates may be hypocellular and misinterpreted as a benign salivary cyst. Centrally placed nuclei, less demarcated cell borders and lack of association with adipocytes may be helpful in distinguishing AcCC with prominent serous acinar cell differentiation from normal acini, [18] and the presence of large nuclei with distinct nucleoli and binucleated cells are further alerting features [67]. FNAC diagnosis of AcCCs entirely composed of non-descript cells lacking secretory granules is more difficult. The accuracy of FNAC seems higher for AcCC with high-grade transformation (see “Pathology”) [64], but this likely reflects detection of obviously malignant cells rather than identification of conventional AcCC. USCB has been less explored, but further immunohistochemistry (see below) can be applied to the material thus obtained (Fig. 3b); a modest 50 % accuracy may be obtained with frozen sections of open biopsies [66].
Pathology
This remains the gold standard for the diagnosis of AcCC.
Macroscopical appearances
Salivary AcCCs are often rounded, circumscribed, and even variously encapsulated masses [18, 68]. Lobulation can be seen, whereas infiltrative growth into adjacent tissues is uncommon. Pending on site, ACCs often range from <1.0 to 4.0 cm. Sizes up to 13.0 cm have been reported [18], possibly reflecting delayed diagnosis or neglected cases. Tumors of minor salivary glands are usually smaller due to earlier detection. Upon dissection, AcCCs are rubbery and solid or variably cystic (Fig. 4). Solid areas are grayish-white or tan with areas of hemorrhage. Necrosis together with multiple, variously separated masses and infiltrative qualities are not uncommon in recurrences [4, 68].
Histology
This is outlined in Table 1. The following discussion is based in standard references and personal experience [3, 9, 11, 12, 14, 15, 17, 18, 68–70].
On routinely prepared sections of resection specimens examined at scanning magnification, AcCCs are variously solid or cystic growths that appear hematoxyphilic or eosinophilic (Fig. 5), the latter influenced by the serous:non-serous cell phenotype ratios and/or proportion of fibrous stroma. Although the tumors are usually non-encapsulated and asymmetrical, they are often lobulated and variously circumscribed; even a fibrous capsule may be seen. Encapsulated, hematoxyphilic tumors would account for Nasse’s ‘blue dot tumor’ [5], and erroneous diagnoses of serous cell or acinar adenoma.
Scanned histological section of AcCC (T) of the palate; the asymmetrical, lobulated, largely solid and hematoxyphilic (purplish) tumor appears stemming from a main duct opening onto surface epithelium (E) (rectangled area); and expands the space between lamina propia (asterisk), skeletal muscle (M), palatine glands (G) and tonsil (Ton), but does not extend therein (a). Asymmetrical, lobulated AcCC (T) partly centred on superficial parotid (P); though largely solid, the tumor appears less hematoxyphilic than that in (a) because of increased, eosinophilic (pink) fibrous stroma (asterisk) (b). Largely cystic AcCC (T) of the parotid (P), which appears less defined than that in (b); the variably sized cysts contain variously inspissated, eosinophilic or amphophilic, secretory material
Tumors of minor salivary glands are centered in the submucosa where they are partly surrounded by salivary lobules; they may involve main ducts (Fig. 5a). Similar to mucoepidermoid carcinoma [2], ‘flooding’ of the lamina propria is unusual. Parotid AcCCs are often superficially located—hence, partly surrounded by glandular lobes (Fig. 5b, c).
Figure 5 also allows appreciation of various silhouettes of AcCC. Tumors irregularly penetrating salivary lobules, soft tissues or bone, ‘satellites’ invading far ahead of the main growth, perineurial invasion and necrosis are not frequent.
Various patterns of growth can be seen. They include solid, microcystic, follicular, papillary (Fig. 6), and cystic (Fig. 5c); and occur alone or in combination [17, 71].
Solid growth pattern of serous-like, tumor cells in acinous arrangements; note the subplasmalemmal, dense nuclei (arrowhead) and hematoxyphilic cytoplasm with vacuoles (arrows); interstitial stroma is minimal (a). Microcystic growth pattern; the arrowheads outline a large aggregate of non-serous tumor cells surrounding multiple, small, variably rigid lumina containing eosinophilic secretion (L); comparison with (a) allows appreciation of differences in size, cytoplasmic hue and nuclear position/chromatin pattern between serous and non-serous tumor cells (b). Follicular growth pattern; small luminal structures, often rigid and lined by non-serous cells, contain amphophilic, secretory material with peripheral bubbling as in thyroid follicles (c). Papillary growth pattern; papillations/tufts of non-serous cells are supported by hyperaemic cores (d)
About a quarter of AcCCs are solid with an easily recognized histology. The tumor parenchyma therein is organized in packed aggregates, largely of differentiated, serous-like cells laden with hematoxyphilic, secretory granules (Fig. 6a). The solid pattern is characterized by sheets of cells separated by thin fibrovascular strands, and thus often appears trabecular to acinar. When small lumina are formed between the cells, the pattern becomes microcystic (lattice-like). Table 2 compares this conventional subtype of AcCC with normal parotid; some of the features are further described below (see “Histochemistry and electron microscopy” and “Immunohistochemistry and related modalities”). It is noted that occasional intercalated duct-like structures may be irregularly/asymmetrically mixed with serous cell aggregates in conventional AcCCs.
About 77 % of AcCCs are non-solid variants composed of varying proportions of non-serous cells in microcystic, cystic, follicular, and papillary architectural arrangements (Figs. 5c, 6b–d), which often present diagnostic difficulties for the non-specialist. For instance, follicular AcCC with its follicular-like structures, lined by cuboidal or flattened epithelial cells containing colloid-like secretion [71], may resemble thyroid carcinoma; small papillary AcCC could be mistaken as papillary cystadenoma. The non-serous cells are traditionally regarded as intercalated duct-like cells. They are small, cuboidal with indistinct borders, scant eosinophilic cytoplasm and central, lightly stained nuclei with inconspicuous nucleoli (Fig. 7); denser nuclei are associated with follicular arrangements.
Cytoplasmic vacuoles (arrows) of non-serous cells. Compare with Fig. 6a
Both serous and non-serous cells may show characteristic cytoplasmic ‘vacuoles’ that probably reflect cytoplasmic lumina (see “Histochemistry and electron microscopy” section (Figs. 6a, 7) and are diagnostically useful. They may coalesce in AcCCS composed of non-serous cells, which results in true lumina and microcystic areas eventually.
In our opinion, the presence of ‘clear’ cells in AcCC has been overemphasized. Certainly ‘pale’ cells are a feature of AcCC (Fig. 8a), but they are not extensive and their cytoplasmic qualities are not those of the ‘empty’ appearing clear cells of mucoepidermoid carcinoma [2]. On these grounds, purported difficulties in distinguishing ‘clear’ cell AcCC from hyalinizing clear cell carcinoma or epithelial-myoepithelial carcinoma do not seem justified.
Rounded collections of pale cells showing faintly hematoxyphilic, fine granules in a clear cytoplasm (a). Apocrine features of adluminal columnar cells; they show eosinophilic cytoplasm and intraluminally bulging apex (arrowhead); lymphocytes and extravasated erythrocytes are present in the lumen (b). Mucous cells with subplasmalemmal nuclei and bubbly hematoxyphilic cytoplasm (arrowheads) in an aggregate largely composed of non-serous tumor cells and variably collapsed small lumina (c)
Apocrine and mucous phenotypes (Fig. 8), mitoses, microliths [72], and iron uptake/storage (Fig. 9) are occasionally seen in AcCC. Iron uptake/storage may be diagnostically useful, but can be seen in salivary adenomas as well [73]. Similar to mucoepidermoid carcinoma [2], stromal lymphoid aggregates/benign lymphopoiesis and cholesterol granulomas are features of AcCC (Fig. 10). The former may be conspicuous and is well established—hence, attempts at defining a novel ‘Warthinoid’ subtype of AcCC do not seem justified.
Collections of heavily pigmented/hemosiderin laden tumor cells (arrowheads) in an AcCC of the parotid; a nerve fascicle (N) is seen between the pushing tumor and normal gland (a). Perls special staining allows better appreciation of the extent of intracellular hemosiderin in an AcCC with papillary growth pattern (b)
The characterisation ‘de-differentiated’ or, preferably, ‘AcCC with high-grade transformation’ is used when a typical low-grade AcCC, primary or metastatic, shows areas resembling high-grade adenocarcinoma (Fig. 11) or undifferentiated carcinoma (including small cell carcinoma types). These lesions may reflect histologic progression and cannot be identified without areas of typical AcCC appearance [23, 25, 74]. Whenever dedifferentiation or undifferentiated areas are observed, clinical outcome is significantly worse, as reflected by the finding of lymph node metastasis or the development of distant disease, with about two-thirds of patients dying from disease after a median of 4.3 years [25].
Similar to mucoepidermoid carcinoma [2], AcCC shows little or no epithelial-mesenchymal transdifferentiation (EMT) [75].
Histologic features of prognostic significance and grading
Histologic features of AcCC that may influence prognosis include: size; silhouette/tumor delineation (circumscribed versus infiltrative); stromal lymphoid aggregates/tumor associated lymphoid proliferation (TALP); necrosis; mitotic rate determined by examining ten high-power fields (HPFs) in areas of greatest concentration of mitoses; atypical mitoses; nuclear pleomorphism; extension beyond the glandular capsule, although this is inapplicable to tumors of non-encapsulated minor salivary glands; vascular and perineural invasion; status of resection margins and regional lymph nodes; and proliferative index usually determined by immunohistochemistry for the Ki-67 antigen (also see “Immunohistochemsitry and related modalities” section) (Fig. 12) [30, 32]. Controversy surrounds the possible significance of architectural arrangements. Spiro et al. [12] suggested that papillary/cystic patterns are associated with worse survival, but this was not confirmed by others [13, 32, 35, 71]. Overall, especially adverse histologic features indicating worse overall/disease-free survival and loco-regional control (see “Prognosis” and Table 4 therein) are positive resection margins, extracapsular extension, vascular/perineural invasion, necrosis, nuclear pleomorphism, high mitotic rate (>2/10 HPFs), atypical mitoses and a Ki-67 index >5 % [30, 32]. Low Ki-67 (<5 %) correlate with TUNEL (terminal deoxynucleotidyl transferase [TdT]-mediated dUTP-biotin nick end labeling: identification of DNA breaks in apoptotic cells) positivity and a good prognosis [76, 77].
In contrast with adenoid cystic carcinoma and mucoepidermoid carcinoma, the WHO has not suggested a histologic grading system for AcCC [3]. Features mentioned above would be useful in constructing such a system. Recently, a ‘proliferative grading system’ for AcCC has been suggested, which distinguishes high- and low-grade tumors based on the presence of an increased mitotic rate (>2 mitoses/10 HPFs), necrosis and presence of pleomorphic cells in combination with extracapsular extension and positive resection margins. Using this system, the authors classified 35 % of AcCC as high-grade [32]. Histologic grading is of significance since high-grade AcCC seems associated with advanced stage disease, higher incidence of distant metastasis and poorer outcome [27, 32, 71, 78]. A population-based study analyzed the prognostic effect of histological grade of AcCC and reported that patients with low-, moderate or high-grade tumors showed a 20-year survival of 98, 83, and 38 %, respectively [28]. Grading would also be useful for individualizing treatment; high-grade tumors would opt for high intensity management. While additional radiotherapy may be considered for high-grade tumors, patients with low-grade tumors would be spared from the morbidity of such intensified treatment [32].
Histochemistry and electron microscopy
Conventional histochemical investigations of AcCC are mainly concerned with demonstrating mucosubstances in the cytoplasmic granules of tumor cells. The periodic acid-Schiff (PAS) positive reaction of those granules, indicative of the presence of neutral glycoproteins, was firstly reported by Godwin et al. [4] and is established. The presence of acidic glycoproteins in AcCC is far less appreciated, although the classic study by Abrams et al. [9] noted variable staining of tumor cells with aldehyde fuchsin and Alcian Blue (AB), indicative of such glycoproteins. Figure 13 illustrates patterns of mucosubstance distribution in AcCC. Staining with tannic acid-phosphomolybdic acid-Levanol fast cyanine 5RN (TPL), a technique used for demonstrating normal salivary myoepithelial cells, is not seen [79]. This would accord with the inconspicuous EMT in AcCC [75].
Echevarria’s early electron microscopical investigation reported on the effects of preservation in effecting ‘clear’ cell phenotypes and illustrated complex phagosomes in tumor cells [80]. Later investigations using material specifically preserved for electron microscopy described variations in the electron density of the cytoplasmic granules in tumor cells [80–82]. These granules (Fig. 14) usually lack the complex polypartite substructure characterizing the secretory granules of normal salivary glands [83], but preservation nuances should be considered before conclusions are drawn. The presence of phagosomes in tumor cells has been confirmed [82, 84], and can be attributed to lysosomal events and phagy. This is supported by an electron microscopical cytochemical investigation of AcCC, which demonstrated lysosomal enzyme activity in tumor cells laden with secretory granules [85]. Recent electron microscopical investigations illustrate occasional ‘myoepithelial’ cells at the periphery of tumor cell aggregates [82, 84]. This should not detract from the notion of inconspicuous EMT in AcCC, as limited sampling is an inherent limitation of electron microscopy and the observations are inconsistent with the results of TPL-histochemistry [79] and immunohistochemistry (see below). Dardick et al. [84] paid particular attention to the microcystic architectural arrangements in AcCC and interpreted them as intercalated duct-like structures. Chaudhry et al. [82] were able to provide electron micrographs of cytoplasmic lumina in tumor cells, which support their correspondence with the histologically seen cytoplasmic vacuoles. Myosin adenosine triphosphatase activity relates to formation of lumina in mammalian salivary glands [86] and an electron microscopical cytochemical investigation of that enzyme in AcCC would be thus of interest. Secretory granules suggestive of neuroendocrine differentiation have been electron microscopically demonstrated in an AcCC of the parotid [87].
Electron micrograph of serous-like cell in AcCC fixed in glutaraldehyde/osmium tetroxide and stained with uranyl acetate and lead citrate. Variously dense secretory granules are at the left part of the rounded nucleus. Mitochondria and rough endoplasmic reticulum are at the right part. The arrow indicates a phagosome. Golgi complex (G)
Immunohistochemistry and related modalities
The literature is extensive and as in mucoepidermoid carcinoma, the WHO refrains from attempting a meaningful review [2, 3]. The present article is not intended as a conventional review of the immunohistochemistry of AcCC and the following brief discussion is based on references selected in view of the various arguments.
Studies concerned with comparing expression of various secretory components between AcCC and normal salivary glands, reported variable amylase, lactoferrin, secretory piece and proline-rich protein immunoreactivities in the tumor, whereas lysozyme is rarely expressed [88]. Notably, despite histologic similarities between normal serous acini and serous-like tumor cells, amylase is not regularly expressed in AcCC.
The results of immunohistochemically assessing cytoskeleton/cytoplasmic filaments correspond with the patterns of tumor differentiation (see “Histogenesis and animal models”). Cytokeratin (CK) ‘cocktails’ are gradually superseded by staining for individual CKs. The basic CK7 and acid CK19, which are of low molecular weight and reflect simple glandular phenotypes, are often expressed in AcCCs of intercalated duct-like cells in microcystic, cystic, follicular or papillary architectural arrangements; based on CK7 staining and analogous to CK7 expression in normal salivary gland epithelia, three distinct histogenetic subtypes of AcCC are recognized: acinar differentiation as seen in blue dot tumors (CK7-negative), ductal differentiation as seen in papillary-cystic tumors (diffuse CK7-positive) and mixed ductulo-acinar (10–66 % CK7-positive cells) differentiation [71]. Staining for CK7 is not seen in solid AcCCs of serous-like cells [71]. AcCC (both low- and HG components) stain with the same intensity using pankeratin antibodies AE1/AE3 and CK18. Cytokeratins CK5/6, CK7, and CK19 are expressed in low-grade AcCC, but not in HG components. CK14 and CK20 are absent in AcCC [25]. Abundant secretory granules in the latter may, however, effect attenuation/displacement of the cytoskeleton and affect detection of immunoreactivities. Myofilament-associated smooth muscle actin or calponin immunoreactivities have not been reported in AcCC [89], which accords with the results of TPL-histochemistry and inconspicuous EMT in AcCC [75, 79]. Vimentin also seems absent [90].
Novel markers have recently reinforced the particular patterns of differentiation in AcCC. The chloride channel DOG1 (Anoctamin-1, described in gastrointestinal stromal tumors—GIST1), selectively expressed in the luminal plasmalemma of serous acinar and intercalated ductal cells and the transcriptional activator SOX10 expressed in nuclei of those cells, are variously immunolocalized in AcCC [91, 92]. Positive DOG1 staining can be an admixture of apical membranous, cytoplasmic, and complete membranous staining, and would support AcCC versus many differential diagnoses. This differential diagnosis includes MASC, but biphasic tumors like, e.g. adenoid cystic carcinoma and epithelial-myoepithelial carcinoma can also express DOG1, although to a lesser degree and lower intensity than AcCC [91, 93].
Controversy surrounds the expression of plasmalemma-anchored, epithelial membrane antigen (MUC1) in AcCC. While Gusterson et al. [94] did not record any immunoreactivity, later studies reported regular staining [95]. Of other plasmalemmal molecules, AcCC shows variable membranous staining for CD44 and integrin αvβ3 [96].
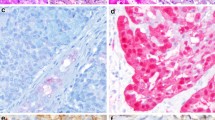
Lysosomal events and phagy in AcCC have already been considered (see “Histochemistry and electron microscopy” section). In this vein, an immunohistochemical study demonstrated widespread, cytoplasmic, and apical expression of CD63, a glycoprotein of lysosomal membranes, in serous-like and non-serous tumor cells, respectively (Fig. 15), and around microliths [97]. The findings can be attributed to lysosomal processing and/or auto-/heterophagy of secretory material.
It is generally regarded that S-100 protein is not expressed in AcCC [98]. The significance of this rather unpretentious feature is now increasingly appreciated (see “Differential diagnosis” and “Proposed classification”).
Immunohistochemistry confirmed aberrant neuroendocrine differentiation in few AcCCs [87, 99–102]. This may result in a paraneoplastic syndrome [103], but is probably a pathological curiosity.
Except for the S-100 protein immunoreactivities, the above features seem largely academic. The following molecular biological aspects can, however, be useful in grading and prognosis.
Molecules associated with the cell cycle are firstly considered. We have already commented on the significance of the Ki67 index (see “Histologic features of prognostic significance and grading”). The Ki-67 index, an independent prognosticator for all SGCs [76, 104, 105], is markedly increased (Ki-67 index up to 60 %) in AcCC with high-grade transformation, where high expression of cyclin D1 is also seen [25]. In contrast with the Ki-67 index, argyrophilic nucleolar organizer region-associated proteins (AgNORs) have not been found of prognostic value in AcCC [106]. Apoptosis assessed by immunohistochemistry for bcl-2 protein and TUNEL, seems more pronounced in Stage I AcCC and overexpression of p53 (nuclear staining >10 %) is low [107]. Recently, the mammalian target of rapamycin (mTOR), significant in the homonymous signaling pathway that regulates cell cycle and promotes proliferation, has been variously immunolocalized in AcCC [108, 109].
Growth factor receptors are now considered. A study using a tissue microarray reported epidermal growth factor receptor (EGFR, HER-1) immunoreactivity in 30 out of 168 AcCCs (17.9 %), which varied from weak to strong [110], whereas a conventional immunohistochemical study reported weak staining in three out of 6 tumors [109]. Overexpression of epidermal growth factor receptor 2 (HER-2/neu, ErbB-2) is less common (one out of 170, 0.59 %) [111]. In situ hybridization, however, suggests that HER-2/neu is overexpressed at the mRNA level in AcCC [112]. In an in vitro situation, targeting overexpressed Her-2 with Gefitinib has resulted in cytostasis in one AcCC derived cell line [113].
Of proteins involved in DNA damage repair, p53 protein is usually not detected in LG components, but strongly expressed (>50 %) in the HG areas of AcCC. P63, a p 53 homologue, has recently been proposed to differentiate AcCC (no expression) from MEC (nearly always positive) [114]. The MEC-specific CRTC1–MAML2 gene fusion is another useful biomarker that distinguishes MEC from AcCC [115].
Little is known about expression of sex hormone receptors in AcCC, which precludes from assessing any therapeutic/prognostic correlations. An institutional study reported immunoreactivity for androgen receptors in two out of ten tumors [116].
Cytophotometry and flow cytometry
In contrast with mucoepidermoid carcinoma [2, 117], cytophotometry quantified DNA does not correlate with the clinical course of AcCC [117, 118]. In addition, prognosis seems similar for tumors with diploid or aneuploid DNA assessed by flow cytometry [106, 119].
Differential diagnosis
We have already alluded to potential problems (see “Histology”). Diagnosis of solid AcCC with largely serous-like cells would not pose significant problems for the aware general pathologist with adequate exposure to salivary pathology while in training. AcCC of non-serous cell in various architectural arrangements is completely the opposite. Even novices in oral/head and neck pathology would experience difficulties and frustration. Detection of cytoplasmic vacuoles would almost be diagnostic for the experienced specialist and other clues (e.g., hemosiderin pigmentation) would be helpful. Standard references offer appropriate advice on the traditional differential diagnosis of AcCC from the perspective of trainees/non-specialists [18, 69] and we further comment on particular aspects. Largely unicystic, AcCC with stromal lymphoid aggregates may be misinterpreted as lymphoepithelial cyst on casual inspection [60, 67], but any invasive growth in the ‘wall’ of the cystic structure and/or intraluminal papillations should be alerting. Difficulties in distinguishing follicular and/or papillary AcCC from salivary metastases of thyroid carcinomas may have been overemphasized; such metastases are rare; when in doubt the characteristic nuclear features of papillary thyroid carcinomas (overlapping, ‘empty’ appearance, grooves, pseudoinclusions) should be sought and immunohistochemistry (thyroglobulin etc.) may not be even necessary. In our opinion, unduly attention has been paid to the role of immunohistochemistry for p63 and CK5/6 in differentiating AcCC from salivary oncocytoma; [120] recognition of oncocytic features is rather straightforward on hematoxylin and eosin stained sections of good quality and solid AcCCs of non-serous, eosinophilic cells are rare. Occasionally, however, there are difficulties in distinguishing microcystic and follicular AcCC from mucoepidermoid carcinoma with inconspicuous mucous/squamoid cells as both tumors may appear variously cystic/papillary with simple, eosinophilic cells and stromal, lymphoid aggregates. In this case, immunohistochemistry for p63 is recommended, as staining would be present in intermediate/non-descript cells of mucoepidermoid carcinoma and usually absent from AcCC [114, 121].
Currently, the greatest diagnostic challenge is differentiating AcCC from MASC. As MASC is histologically similar to microcystic/papillary AcCC of non-serous cells, [21, 22, 122–128] distinction usually relies on special techniques. It has been reported that MASC cells lack PAS-positive secretory granules [122, 126], but this is a matter of dispute. In addition, pathologists trained in the present era, where diagnostic immunohistochemistry and molecular testing reign, may have difficulties in interpreting conventional mucosubstance histochemistry. Immunohistochemistry seems more helpful as staining for vimentin, S-100 protein, proteins related to secretory mechanisms (STAT5a and mammaglobin) and adipophilin (a component of milk lipid globule membranes) is usually positive in MASC, though negative in AcCC [21, 123, 127, 129]. Immunostaining should always be interpreted in conjunction with routine histology as S-100 protein and mammaglobin immunoreactivities are features of other SGCs [130, 131]. In addition, nuclear staining for the transcription factor GATA3 is another feature associated with MASC, but not with conventional AcCC [132]. Caution should also be exerted as regards staining with adipophilin [126] and cross-immunoreactivity with lipid-rich residues of lysosomal events/phagy (see “Histochemistry and electron microscopy” and “Immunohistochemistry and related modalities”) should be considered. A definite diagnosis of MASC can only be established via demonstration of the chromosomal t(12;15) (p13;q25) translocation, which results in fusion between the ETV6 gene on chromosome 12 (a transcription regulator) and the NTRK3 gene on chromosome 15 (a membrane receptor kinase influencing cell proliferation and survival) [126]. This genetic re-arrangement, usually demonstrated by means of ETV6 fluorescence in situ hybridization (FISH) [124, 127, 128], is not found in other SGCs [21, 133–135]. Chiosea et al. [125] detected ETV6 translocation in so-called ‘zymogen granule poor AcCCs’, but they subsequently re-classified them as MASC.
It should be appreciated that FISH and antibodies for mammaglobin or DOG1 may not be available to all pathology laboratories. We therefore recommend that all salivary tumors with a histologic appearance of non-serous, microcystic/papillary AcCC are routinely immunostained for S-100 protein. If staining is negative, a diagnosis of AcCC should be established; if staining is positive and in the absence of more specific tests, the pathologist should raise the possibility of MASC and explain the situation in his/her report. The nuances in distinction may be academic to clinicians as both conventional AcCC and MASC share a similar outcome [125].
Histogenesis and animal models
Although proliferative capacity lies with all types of salivary glandular cells [72], the origin of AcCC has been traditionally sought among purported, ‘semipluripotential reserve’ or ‘stem’ cells located at the acinar-intercalated ductal region of salivary glands; proliferation and abnormal cytodifferentiation of those cells would result in AcCC [7, 23, 136, 137]. Chaudhry et al. [82] attributed ‘pluripotential reserve/stem’ qualities to simple tumor cells with a high nuclear:cytoplasmic ratio and few organelles. Overall interpretations of electron microscopical observations [82, 84] and the immunohistochemical localization of DOG1 and SOX10 in AcCC [92, 94], as discussed above, suggest that the histogenesis of AcCC simulates events at the ends of branching rudiments during salivary embryogenesis. In this regard, we may envisage the histology of AcCC (see above) as a continuum. At one end would be AcCC of simple duct-like cells (intercalated duct-like or incompletely differentiated acinar) in microcystic or other architectural arrangements, whereas at the other end would be solid AcCC of differentiated serous-like cells; AcCC of varying proportions of duct-/serous-like cells possibly occupies a middle position and myoepithelial differentiation is not prominent. Interestingly, the acinar differentiation seems functional as in vitro tumor cells secrete amylase when stimulated by adrenalin [48, 138, 139]. The features in S1 can be reconciled with this histogenetic model; AcCC may spread to involve main ducts and acini emptying into proximal extralobular ducts have been described in mammalian salivary glands [138].
Little is known about naturally occurring, animal models for the study of human salivary tumors. However, 70 % of male transgenic (MMTV/v-Ha-ras) mice develop tumors in their parotids, which show electron microscopic features and immunohistochemical expression of amylase similar to those of AcCC [140]. The model is of interest, but has not enjoyed widespread endorsement and further application. Of greater clinical potential appears the deletion of both the Adenomatous Polyposis Coli (APC) and Phosphatase and tensin homologue (PTEN) tumor suppressor genes in mice, which results in activation of the mTOR pathway and formation of salivary gland tumors resembling human AcCC with 100 % penetrance; treatment with the rapamycin inhibited mTOR and led to complete regression of tumors, which indicates dependence of growth on sustained signaling [141]. The results allow pondering whether treatment with mTOR inhibitors may benefit AcCC patients, given immunohistochemical confirmation of activated mTOR signaling in human AcCC [108, 109].
Genetics
The genetic landscape of AcCC is insufficiently explored. In the Mitelman database of Chromosome Aberrations and Gene Fusions in Cancer there are only 11 cases with cytogenetic alterations published (http://cgap.nci.nih.gov/Chromosomes/Mitelman). Early investigations showed alterations, often loss of heterozygosity, in 84 % out of 25 AcCCs frequently altered regions being on 4p and 17p, followed by 5q and 6p [142]. The only recurrent changes observed are extra copies of chromosome 8 and deletions or translocations with breakpoints in 6q13-q24. Terminal 6q-deletions are a typical feature of all major subtypes of SGCs [143]. A single AcCC showed deletions in the tumor suppressor CDKN2A and proapoptotic cofactor of p53-encoding PPP1R13B; and mutations in the cell growth regulator EP300 [144]. Given the possible significance of the PTEN-activated mTOR signaling pathway (see “Immunohistochemistry and related modalities” and “Histogenesis and animal models”) [108, 109, 141], the sporadic association of AcCC with the effected by germline mutations of PTEN, Cowden syndrome (see “Epidemiology”) [42] is of interest. The situation regarding the ETV6–NTRK3 fusion is still evolving (see “Differential diagnosis” and “Proposed classification”).
Proposed classification
The academically meritorious discovery of ETV6 re-arrangement in tumors histologically similar to microcystic AcCC of non-serous cells and the introduction of the MASC concept (see “Definition and brief historical survey” and “Differential diagnosis”) has excited much interest [21]; [22, 122–128]. There are over 40 related publications in the PubMed data base (http://www.ncbi.nlm.nih.gov/pubmed). The histologic similarities and increased availability of ETV6 FISH prompted institutional re-examination of cases previously diagnosed as AcCC. While non-serous tumors of microcystic and papillary architectural arrangements were thus re-classified as MASC, solid tumors of serous cells did not harbor the characteristic gene re-arrangement [22, 125, 145]. Further corroboration is desirable, but the results suggest re-classifying salivary tumors with MASC and ‘serous cell adenocarcinoma’ (an old term aptly describing solid AcCC of serous-like cells) featuring as distinct entities. Breast pathologists support distinguishing secretory from acinic cell carcinoma [133] and pending rigorous epidemiological testing, MASC and AcCC may show an opposite gender distribution (male-to-female ratio 8:2) as compared to AcCC (male-to-female ratio, 1:1.5) [27, 28, 37, 125]. Alternatively, AcCC lacks a bimodal age pattern (see “Epidemiology”); it may be imprudent playing down histologic and prognostic similarities between AcCC and MASC; [125] a particular genetic alteration may not be detected in every tumor, e.g. the MAML2 re-arrangement is seen in 50–70 % of mucoepidermoid carcinomas; [2] regular expression of DOG1 or SOX10 in every AcCC would be unrealistic; and high-quality electron microscopical investigations of MASC are urgently needed, particularly in view of observations regarding adipophilin immunoreactivity and lysosomal events/phagy (see “Differential diagnosis”) [97, 127]. A sensible compromise in keeping with current knowledge and arguments for and against re-classification is shown in Table 3. The approach, based on the continuum discussed under “Histogenesis and animal models”, suggests a family of tumors rather than distinct entities; is centered on the established term AcCC, though in the modified form of ‘acinic-intercalated ductal carcinoma’ to smooth away the apprehensive notion of AcCCs largely composed of cells lacking obvious secretory granules (see “Definition and brief historical survey”); and would be more palatable to clinicians concerned about intricacies of histologic classifications.
Management
Surgery: the primary tumor
As AcCCs are often anatomically accessible tumors and patients do not show distant metastases at presentation, the treatment of choice would be complete resection aiming at achieving free margins, thereby avoiding post-operative morbidity [35, 54]. AcCC may be, however, initially underestimated, as indicated by the high number of redo-cases in a recent study [36].
Surgery alone will likely be curative for low-grade AcCC. The extent of the operation should parallel the loco-regional anatomical extent of the tumor as influenced by the site of origin. Although superficial parotidectomy often effects complete removal, more extended, conservative parotidectomy is indicated if the deep lobe is involved. A pre-operatively functioning facial nerve can be preserved without loss of oncologic control, even if there would be no margin between tumor and nerve, and any microscopic, residual disease seems treatable with post-operative radiotherapy [54]. A more aggressive, initial approach would be required for locally advanced AcCCs, especially pre-operatively known high-grade tumors in risk of positive margins, bone/nerve invasion, and nodal metastases. A pre-operatively paralyzed or grossly invaded/surrounded facial nerve should be resected and reconstructed with an interposition graft from the greater auricular or sural nerve. Advanced cases may also require resection of skin, posterior mandible/masseter or lateral temporal bone, followed by a free flap reconstruction. In AcCC of minor salivary glands, local anatomy will dictate the best surgical approach [47].
Surgery: the neck
Elective ND in patients with AcCC is usually not recommended because of a relatively low incidence of regional lymph node metastasis (10 %). The MDACC study, however, observing that addition of an ND to the surgical strategy decreases the rate of regional recurrences, suggests that patients with large tumor volume or tumors with high-grade features in the pre-operative biopsy would likely benefit from elective ND of levels II, III and IV [36].
Clinically positive cervical lymph nodes at presentation are an adverse prognosticator necessitating therapeutic ND as part of the surgical approach and should raise suspicion of an AcCC with HG transformation.
Radiotherapy
Low grade, low stage (I and II), and adequately resected AcCCs are not considered for radiotherapy, as their prognosis is excellent with surgery alone [54, 146]. This is supported by a recent SEER analysis specifically assessing any oncologic benefits of additional radiotherapy [147]. The study did not demonstrate an effect of post-operative radiotherapy on stage I and II, low-grade AcCC; no disease-specific deaths were recorded in 50 stage I, low-grade tumors treated with surgery alone.
Criteria for additional radiotherapy do not differ from those for other SGCs [54] and include salvage surgery for recurrent disease; advanced T-classification (T3/T4); positive surgical margins; pathologically positive, cervical lymph nodes; perineural invasion; and high-grade/highly proliferative tumors [28, 32, 36]. Patients with prognostically worse AcCC selected to undergo post-operative radiotherapy through application of those criteria, doubled their chance of staying disease-free when thus treated (HR of 2, p = 0.04) in multivariate analysis).
Conversely, the SEER analysis undertaken by Biron et al. [44] concludes that ‘radiotherapy probably is not effective in AcCC’; the study even suggests and that after multivariate correction for stage and grade, radiotherapy implied a death hazard ratio of 2. Caution should be, however, exerted as it is imprudent to retrospectively assess the value of a ‘treatment’. It is noted that the SEER analysis does not correct for involved resection margins or initially inadequate treatment, which account for a substantial part of AcCC patients [36] and would probably end up being radiated. Missing data in the variables corrected for, are further weakening the conclusions; for instance, although the analysis spanned from 1973 to 2009, precise TNM classification had only been obtained for patients from 2000 to 2005 [44]. In other words, even after corrected “roughly” for stage and grade, significant selection and information bias still is likely present in the retrospective SEER data, resulting from the reality that hard-to-capture prognostic factors have usually been incorporated in a clinical decision to add radiotherapy to the treatment of early stage AcCC that worries the treating oncologist.
Chemotherapy
Little is known regarding chemotherapy in AcCC. The potential value of mTOR inhibitors has already been mentioned (see “Histogenesis and animal models”), but no specific chemotherapeutic agents have been currently approved. Nevertheless, an observed distant metastasis rate of 1 in 5 (most commonly in the lungs) indicates the need for developing such treatment [36].
Prognosis
Endorsing the outlined therapeutic strategies (see “Management”), AcCC is generally considered to have the best survival rate among SGCs, although the subgroup with high-grade transformation has a poorer prognosis [28]; [27, 148, 149]. AcCC is by no means an innocent tumor. Earlier studies reported that the cure rate decreased from 76 to 89 % at 5 years to 55 % at 15 years and 56 % at 20 years [150], similar trends being noted by others [12, 13]. The evidence suggests a protracted clinical course with recurrences occurring years or even decades after initial diagnosis and treatment (mean time to recurrence, 92 months) [32, 35, 66]. Clearly, this can be an aggressive tumor that should be treated accordingly and appropriate initial treatment would thus obviously affect prognosis. Mere enucleation is totally inadequate [12, 151] and this has been recently corroborated by multivariate analysis [36]. The effects of selectively applied post-operative radiotherapy have been discussed above (see “Management”) [54, 146, 152]. A recent institutional study indicates a median survival of 28.5 years, with only 13 out of 155 patients (8.4 %) dying of their disease (mean time to death from disease, 3.8 years; range, 0.7–11.2 years) [36]. Selection/referral bias obviously affects institutional results as large tertiary/referral centers a usually end with prognostically worse cases and a higher proportion of patients with either residual or recurrent disease after suboptimal initial treatment. Nevertheless, better results are now being reported. Recent population-based studies indicate overall 5-, 10-, and 20-year survival of 97, 94, and 90 %, respectively; survival dropped to 22 % in patients with distant metastasis [28].
Prognostic factors considered as significant are shown in Table 4. They include age; [36] pain; [35] gender, AcCC being probably the sole SGC where this appears significant; [28, 36] race, with colored individuals having a worse outcome; [28] previous inadequate treatment; [36] extent of disease (advanced T-classification, invasion of tumor beyond glandular capsule, advanced N classification); [13, 28, 32, 35, 36, 44] and especially invasion of the skull base [153]. In a series from the Mayo clinic, lateral skull base invasion, mainly direct extension of tumor through the stylomastoid foramen, occurred in one of ten patients, in 80 % of these following local recurrence [153]. Of note, following skull base recurrences, also low-grade AcCC had a fatal outcome, and only one in two patients with this feature made it through the next 2 years [153].
Invasion of the anterior skull base is uncommon and typically associated with the rare sinonasal AcCC. Anterior skull base invasion is infrequently seen, typically in the rarely occurring sinonasal AcCC. However, sinonasal origin as such does not seem to carry a worse prognosis, as evidenced from the 18 cases in the SEER database that were matched to major salivary gland AcCC [154]. A general idea of the effect of UICC/AJCC stage on outcome in the largest series reported to date is that Stage I tumors carry a 93.5 % 20 years DSS, Stage II tumors a 98 % 20 years DSS, Stage III tumors and Stage IV tumors a 64 % 20 years DSS [44]. Disease-specific deaths are not uncommon in the course of AcCC; In a recent series, 76.9 % of those were attributable to distant metastases [32].
Of the factors considered above, the MDACC multivariate analysis regards the following factors as influencing overall and disease-free survival in AcCC: gender, inadequate previous treatment (these patients have a significantly higher chance of succumbing to disease and a hazard Ratio of recurrence twice as high as that of advanced vs low stage disease), extent of disease (T-classification, UICC/AJCC stage), positive resection margins and age at diagnosis [36]. With the exception of gender, these factors had already been identified and confirmed in other studies dealing with all types of SGCs [26, 155, 156]. Gender did not feature as a significant prognostic factor in the SEER analysis of Biron et al. [44]. In the latter analysis, the factors remaining in multivariate analysis were advanced stage (HR 2), minor salivary gland subsite (HR 3) and HG (HR 3.3 for grade III and 8.1 for grade IV).
Epilog
Our perception of salivary AcCC has been repeatedly modified over the 12 decades since its description. Successes include the introduction of modern imaging modalities in the assessment of patients; application of various morphological methodologies to characterize cellular phenotypes/events suggestive of distorted embryonic development; and multivariate analyses of population-based datasets/institution-based series indicative of factors influencing prognosis, management, and outcome. In addition, molecular methodologies introduced the concept of MASC and prompted further thinking and research. Uncertainties, however, remain. Links between particular genetic alterations and cellular phenotypes reflecting abnormal events at the ends of branching salivary rudiments should be explored; the role of the S-100 protein in salivary pathobiology should be clarified; and high-grade transformation and patterns of nodal metastasis should be precisely characterized. Prospects are good, but would require continuous research efforts in the hope that non-invasive therapies and gene manipulation may become available in future.
References
Coca-Pelaz A, Rodrigo JP, Bradley PJ, Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A, Haigentz M Jr, Takes RP, Mondin V, Teymoortash A, Thompson LD, Ferlito A (2015) Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol 51:652–661
Coca-Pelaz A, Rodrigo JP, Triantafyllou A, Hunt JL, Rinaldo A, Strojan P, Haigentz M Jr, Mendenhall WM, Takes RP, Vander Poorten V, Ferlito A (2015) Salivary mucoepidermoid carcinoma revisited. Eur Arch Otorhinolaryngol 272:799–819
Ellis G, Simpson RHW (2005) Acinic cell carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D (eds) Pathology and genetics of head and neck tumours. IARC Press, Lyon, pp 219–220
Godwin JT, Foote FW Jr, Fazell EL (1954) Acinic cell adenocarcinoma of the parotid gland; report of twenty-seven cases. Am J Pathol 30:465–477
Nasse D (1892) Die Geschwülste Der Speicheldrüsen Und VerwandteTumoren Des Kopfes. Arch Klin Chir 44:233–302
Evans RW, Cruickshank AH (1970) Epithelial tumours of the salivary glands. Saunders, Philadelphia
Buxton RW, Maxwell JH, French AJ (1953) Surgical treatment of epithelial tumors of the parotid gland. Surg Gynecol Obstet 97:401–416
Foote FW Jr, Frazell EL (1954) Salivary gland tumors. In: Armed Forces Institute of Pathology (ed) Atlas of tumor pathology. AFIP, Washington
Abrams AM, Cornyn J, Scofield HH, Hansen LS (1965) Acinic cell adenocarcinoma of the major salivary glands. A clinicopathologic study of 77 cases. Cancer 18:1145–1162
Thackray AC, Sobin LH (1972) Histological typing of salivary gland tumours. In: World Health Organization (ed) World Health Organization international histological classification of tumours. World Health Organization, Geneva
Thackray AC, Lucas RB (1974) Tumors of the major salivary glands. Atlas of tumor pathology, second series, fascicle 10. Armed Forces Institute of Pathology (AFIP), Washington, DC
Spiro RH, Huvos AG, Strong EW (1978) Acinic cell carcinoma of salivary origin. A clinicopathologic study of 67 cases. Cancer 41:924–935
Perzin KH, Livolsi VA (1979) Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer 44:1434–1457
Batsakis JG, Chinn EK, Weimert TA, Work WP, Krause CJ (1979) Acinic cell carcinoma: a clinicopathologic study of thirty-five cases. J Laryngol Otol 93:325–340
Abrams AM, Melrose RJ (1978) Acinic cell tumors of minor salivary gland origin. Oral Surg Oral Med Oral Pathol 46:220–233
Chen SY, Brannon RB, Miller AS, White DK, Hooker SP (1978) Acinic cell adenocarcinoma of minor salivary glands. Cancer 42:678–685
Ellis GL, Corio RL (1983) Acinic cell adenocarcinoma. A clinicopathologic analysis of 294 cases. Cancer 52:542–549
Ellis GL, Auclair PL (1996) Armed Forces Institute of Pathology, Washington DC
Cheuk W, Chan JK (2007) Advances in salivary gland pathology. Histopathology 51:1–20
Simpson RH, Skalova A, Di PS, Leivo I (2014) Recent advances in the diagnostic pathology of salivary carcinomas. Virchows Arch 465:371–384
Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, Starek I, Geierova M, Simpson RH, Passador-Santos F, Ryska A, Leivo I, Kinkor Z, Michal M (2010) Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 34:599–608
Bishop JA, Yonescu R, Batista D, Eisele DW, Westra WH (2013) Most nonparotid “acinic cell carcinomas” represent mammary analog secretory carcinomas. Am J Surg Pathol 37:1053–1057
Batsakis JG, Luna MA, El-Naggar AK (1990) Histopathologic grading of salivary gland neoplasms: II. Acinic cell carcinomas. Ann Otol Rhinol Laryngol 99(11):929–933
Varsegi MF, Ravis SM, Hattab EM, Henley JD, Billings SD (2008) Widespread cutaneous metastases from acinic cell carcinoma 20 years after primary presentation. J Cutan Pathol 35(6):591–593
Skalova A, Sima R, Vanecek T, Muller S, Korabecna M, Nemcova J, Elmberger G, Leivo I, Passador-Santos F, Walter J, Rousarova M, Jedlickova K, Curik R, Geierova M, Michal M (2009) Acinic cell carcinoma with high-grade transformation: a report of 9 cases with immunohistochemical study and analysis of TP53 and HER-2/Neu genes. Am J Surg Pathol 33:1137–1145
Vander Poorten VL, Hart AA, van der Laan BF, Baatenburg de Jong RJ, Manni JJ, Marres HA, Meeuwis CA, Lubsen H, Terhaard CH, Balm AJ (2003) Prognostic index for patients with parotid carcinoma: external validation using the nationwide 1985–1994 Dutch Head and Neck Oncology Cooperative Group Database. Cancer 97:1453–1463
Hoffman HT, Karnell LH, Robinson RA, Pinkston JA, Menck HR (1999) National cancer data base report on cancer of the head and neck: acinic cell carcinoma. Head Neck 21:297–309
Patel NR, Sanghvi S, Khan MN, Husain Q, Baredes S, Eloy JA (2014) Demographic trends and disease-specific survival in salivary acinic cell carcinoma: an analysis of 1129 cases. Laryngoscope 124:172–178
Greig SR, Chaplin JM, McIvor NP, Izzard ME, Taylor G, Wee D (2008) Acinic cell carcinoma of the parotid gland: Auckland experience and literature review. ANZ J Surg 78:754–758
Lin WN, Huang HC, Wu CC, Liao CT, Chen IH, Kan CJ, Huang SF (2010) Analysis of acinic cell carcinoma of the parotid gland—15 years experience. Acta Otolaryngol 130:1406–1410
Omlie JE, Koutlas IG (2010) Acinic cell carcinoma of minor salivary glands: a clinicopathologic study of 21 cases. J Oral Maxillofac Surg 68:2053–2057
Gomez DR, Katabi N, Zhung J, Wolden SL, Zelefsky MJ, Kraus DH, Shah JP, Wong RJ, Ghossein RA, Lee NY (2009) Clinical and pathologic prognostic features in acinic cell carcinoma of the parotid gland. Cancer 115:2128–2137
Van Eycken L (2008) Head and neck cancer. In: Van Eycken L (ed) Cancer incidence in Belgium 2004–2005. Belgian Cancer Registry, Brussels, p 41
Al-Mamgani A, van Rooij P, Verduijn GM, Meeuwis CA, Levendag PC (2012) Long-term outcomes and quality of life of 186 patients with primary parotid carcinoma treated with surgery and radiotherapy at the Daniel Den Hoed Cancer Center. Int J Radiat Oncol Biol Phys 84:189–195
Lewis JE, Olsen KD, Weiland LH (1991) Acinic cell carcinoma. clinicopathologic review. Cancer 67:172–179
Neskey DM, Klein JD, Hicks S, Garden AS, Bell DM, El-Naggar AK, Kies MS, Weber RS, Kupferman ME (2013) Prognostic factors associated with decreased survival in patients with acinic cell carcinoma. JAMA Otolaryngol Head Neck Surg 139:1195–1202
Boukheris H, Curtis RE, Land CE, Dores GM (2009) Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: a population-based study in the United States. Cancer Epidemiol Biomark Prev 18:2899–2906
Yoshida EJ, Garcia J, Eisele DW, Chen AM (2014) Salivary gland malignancies in children. Int J Pediatr Otorhinolaryngol 78:174–178
Callender DL, Frankenthaler RA, Luna MA, Lee SS, Goepfert H (1992) Salivary gland neoplasms in children. Arch Otolaryngol Head Neck Surg 118:472–476
Saku T, Hayashi Y, Takahara O, Matsuura H, Tokunaga M, Tokunaga M, Tokuoka S, Soda M, Mabuchi K, Land CE (1997) Salivary gland tumors among atomic bomb survivors, 1950–1987. Cancer 79:1465–1475
Depowski PL, Setzen G, Chui A, Koltai PJ, Dollar J, Ross JS (1999) Familial occurrence of acinic cell carcinoma of the parotid gland. Arch Pathol Lab Med 123:1118–1120
Villeneuve H, Tremblay S, Galiatsatos P, Hamel N, Guertin L, Morency R, Tischkowitz M (2011) Acinic cell carcinoma of the retromolar trigone region: expanding the tumor phenotype in Cowden syndrome? Fam Cancer 10:691–694
Ripamonti CB, Colombo M, Mondini P, Siranoush M, Peissel B, Bernard L, Radice P, Carcangiu ML (2013) First description of an acinic cell carcinoma of the breast in a BRCA1 mutation carrier: a case report. BMC Cancer 13:46
Biron VL, Lentsch EJ, Gerry DR, Bewley AF (2015) Factors influencing survival in acinic cell carcinoma: a retrospective survival analysis of 2061 patients. Head Neck 37:870–877
Ilayaraja V, Prasad H, Anuthama K, Sruthi R (2014) Acinic cell carcinoma of minor salivary gland showing features of high-grade transformation. J Oral Maxillofac Pathol 18:97–101
Triantafillidou K, Iordanidis F, Psomaderis K, Kalimeras E (2010) Acinic cell carcinoma of minor salivary glands: a clinical and immunohistochemical study. J Oral Maxillofac Surg 68:2489–2496
Vander Poorten V, Hunt J, Bradley PJ, Haigentz M, Rinaldo A, Mendenhall WM, Suarez C, Silver C, Takes RP, Ferlito A (2014) Recent trends in the management of minor salivary gland carcinoma. Head Neck 36:444–455
Neto AG, Pineda-Daboin K, Spencer ML, Luna MA (2005) Sinonasal acinic cell carcinoma: a clinicopathologic study of four cases. Head Neck 27:603–607
Boscolo-Rizzo P, da Mosto MC, Marchiori C, Boccato P (2004) Transglottic acinic cell carcinoma. case report and literature review. ORL J Otorhinolaryngol Relat Spec 66:286–289
Slater L (2013) Bilateral multifocal parotid tumors: acinic cell carcinomas versus nodular oncocytic hyperplasia. J Oral Maxillofac Surg 71:655
Gnepp DR, Schroeder W, Heffner D (1989) Synchronous tumors arising in a single major salivary gland. Cancer 63:1219–1224
Jia YL, Bishwo SP, Nie X, Chen LL (2012) Synchronous bilateral multifocal acinic cell carcinoma of parotid gland: case report and review of the literature. J Oral Maxillofac Surg 70:e574–e580
Chang ED, Lee EJ, Lee AW, Kim JS, Kang CS (2011) Primary acinic cell carcinoma of the breast: a case report with an immunohistochemical and ultrastructural studies. J Breast Cancer 14:160–164
Vander Poorten V, Bradley PJ, Takes RP, Rinaldo A, Woolgar JA, Ferlito A (2012) Diagnosis and management of parotid carcinoma with a special focus on recent advances in molecular biology. Head Neck 34:429–440
Spiro RH (1998) Management of malignant tumors of the salivary glands. Oncology (Huntingt) 12:671–683
Eisele DW, Kleinberg LR, O’Malley BB (1999) Management of malignant salivary gland tumors. In: Harrison LB, Sessions RB, Hong KH (eds) Head and neck cancer. A multidisciplinary approach. Lippincott-Raven, Philadelphia-New York, pp 721–748
Yousem DM, Kraut MA, Chalian AA (2000) Major salivary gland imaging. Radiology 216:19–29
Cheung RL, Russell AC, Freeman J (2008) Does routine preoperative imaging of parotid tumours affect surgical management decision making? J Otolaryngol Head Neck Surg 37:430–434
Li J, Gong X, Xiong P, Xu Q, Liu Y, Chen Y, Tian Z (2014) Ultrasound and computed tomography features of primary acinic cell carcinoma in the parotid gland: a retrospective study. Eur J Radiol 83:1152–1156
Suh SI, Seol HY, Kim TK, Lee NJ, Kim JH, Kim KA, Woo JS, Lee JH (2005) Acinic cell carcinoma of the head and neck: radiologic-pathologic correlation. J Comput Assist Tomogr 29:121–126
Jungehuelsing M, Sittel C, Fischbach R, Wagner M, Stennert E (2000) Limitations of magnetic resonance imaging in the evaluation of perineural tumor spread causing facial nerve paralysis. Arch Otolaryngol Head Neck Surg 126:506–510
Lee YY, Wong KT, King AD, Ahuja AT (2008) Imaging of salivary gland tumours. Eur J Radiol 66:419–436
Hyun OJ, Yoo I, Jung CK, Hoon KS, Chung SK (2010) F-18 FDG PET/CT findings of dedifferentiated acinic cell carcinoma. Clin Nucl Med 35:473–474
Johnykutty S, Miller CH, Hoda RS, Giampoli EJ (2009) Fine-needle aspiration of dedifferentiated acinic cell carcinoma: report of a case with cyto-histological correlation. Diagn Cytopathol 37:763–768
Stanley RJ, Weiland LH, Olsen KD, Pearson BW (1988) Dedifferentiated acinic cell (acinous) carcinoma of the parotid gland. Otolaryngol Head Neck Surg 98:155–161
Cha W, Kim MS, Ahn JC, Cho SW, Sunwoo W, Song CM, Kwon TK, Sung MW, Kim KH (2011) Clinical analysis of acinic cell carcinoma in parotid gland. Clin Exp Otorhinolaryngol 4:188–192
Mosunjac MB, Siddiqui MT, Tadros T (2009) Acinic cell carcinoma-papillary cystic variant. Pitfalls of fine needle aspiration diagnosis: study of five cases and review of literature. Cytopathology 20:96–102
Foote FWJ, Frazell EL (1953) Tumors of the major salivary glands. Cancer 6:1065–1133
Dardick I (1996) Color atlas/text of salivary gland tumor pathology. Igaku-Shoin, New York
Cheuk W, Chan JK (2007) Salivary gland tumors. In: Fletcher C (ed) Diagnostic histopathology of tumors, 4th edn. Elsevier Churchill Livingstone, London, pp 239–325
Schwarz S, Zenk J, Muller M, Ettl T, Wunsch PH, Hartmann A, Agaimy A (2012) The many faces of acinic cell carcinomas of the salivary glands: a study of 40 cases relating histological and immunohistological subtypes to clinical parameters and prognosis. Histopathology 61:395–408
Triantafyllou A, Hunt JL, Devaney KO, Ferlito A (2014) A perspective of comparative salivary and breast pathology. part I: microstructural aspects, adaptations and cellular events. Eur Arch Otorhinolaryngol 271:647–663
Triantafyllou A, Coulter P, Scott J (1999) Phenotypes in canalicular adenoma of human minor salivary glands reflect the interplay of altered secretory product, absent neuro-effector relationships and the diversity of the microenvironment. Histopathology 35:502–516
Jain A, Alam K, Misra A, Maheshwari V (2013) Dedifferentiated acinic cell tumour: the harlequin of salivary gland neoplasms–an unusual variant. BMJ Case Rep. doi:10.1136/bcr-2012-008434
Woolgar J, Triantafyllou A (2011) Contemporary salivary clinical pathology: facts and dilemmas. In: Bradley PJ, Guntinas-Lichius O (eds) Salivary gland disorders and diseases: diagnosis and management. Thieme, Stuttgart, pp 27–41
Skalova A, Leivo I, von Boguslawsky K, Saksela E (1994) Cell proliferation correlates with prognosis in acinic cell carcinomas of salivary gland origin. Immunohistochemical study of 30 cases using the MIB 1 antibody in formalin-fixed paraffin sections. J Pathol 173:13–21
Hellquist HB, Sundelin K, Di BA, Tytor M, Manzotti M, Viale G (1997) Tumour growth fraction and apoptosis in salivary gland acinic cell carcinomas. Prognostic implications of Ki-67 and Bcl-2 expression and of in situ end labelling (TUNEL). J Pathol 181:323–329
Seifert G, Sobin LH (1992) The World Health Organization’s histological classification of salivary gland tumors. A commentary on the second edition. Cancer 70:379–385
Nikai H, El-Bardaie AM, Takata T, Ogawa I, Ijuhin N (1986) Histologic evaluation of myoepithelial participation in salivary gland tumors. Int J Oral Maxillofac Surg 15:597–605
Echevarria RA (1967) Ultrastructure of the acinic cell carcinoma and clear cell carcinoma of the parotid gland. Cancer 20:563–571
Erlandson RA, Tandler B (1972) Ultrastructure of acinic cell carcinoma of the parotid gland. Arch Pathol 93:130–140
Chaudhry AP, Cutler LS, Leifer C, Satchidanand S, Labay G, Yamane G (1986) Histogenesis of acinic cell carcinoma of the major and minor salivary glands. An ultrastructural study. J Pathol 148:307–320
Harrison JD, Auger DW, Badir MS, Paterson KL (1987) Ultrastructural morphology of secretory granules of submandibular and parotid salivary glands of man. Arch Oral Biol 32:229–234
Dardick I, George D, Jeans MT, Wittkuhn JF, Skimming L, Rippstein P, van Nostrand AW (1987) Ultrastructural morphology and cellular differentiation in acinic cell carcinoma. Oral Surg Oral Med Oral Pathol 63:325–334
Franzen L, Carlsoo B, Angstrom T (1979) Cytology and cytochemistry of acinic cell carcinoma. Acta Otolaryngol Suppl 360:174–177
Cutler LS, Mooradian BA (1987) Lumen formation during development of the rat submandibular gland. J Dent Res 66:1559–1562
Ito K, Kakudo K, Mori I, Horiuchi M, Osamura Y (1990) Neuroendocrine differentiation in a case of acinic cell carcinoma of the parotid gland. Acta Pathol Jpn 40:279–287
Warner TF, Seo IS, Azen EA, Hafez GR, Zarling T (1985) Immunocytochemistry of acinic cell carcinomas and mixed tumors of salivary glands. Cancer 56:2221–2227
Prasad AR, Savera AT, Gown AM, Zarbo RJ (1999) The myoepithelial immunophenotype in 135 benign and malignant salivary gland tumors other than pleomorphic adenoma. Arch Pathol Lab Med 123:801–806
Seifert G, Caselitz J (1983) Tumor markers in parotid gland carcinomas: immunohistochemical investigations. Cancer Detect Prev 6:119–130
Chenevert J, Duvvuri U, Chiosea S, Dacic S, Cieply K, Kim J, Shiwarski D, Seethala RR (2012) DOG1: a novel marker of salivary acinar and intercalated duct differentiation. Mod Pathol 25:919–929
Ohtomo R, Mori T, Shibata S, Tsuta K, Maeshima AM, Akazawa C, Watabe Y, Honda K, Yamada T, Yoshimoto S, Asai M, Okano H, Kanai Y, Tsuda H (2013) SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol 26:1041–1050
Hellquist H, Skalova A (2014) Acinic cell carcinoma. In: Hellquist H, Skalova A (eds) Histopathology of the salivary glands. Springer, Heidelberg, pp 261–281
Gusterson BA, Lucas RB, Ormerod MG (1982) Distribution of epithelial membrane antigen in benign and malignant lesions of the salivary glands. Virchows Arch A Pathol Anat Histol 397:227–233
Mannweiler S, Beham A, Langner C (2003) MUC1 and MUC2 expression in salivary gland tumors and in non-neoplastic salivary gland tissue. APMIS 111:978–984
Fok TC, Lapointe H, Tuck AB, Chambers AF, Jackson-Boeters L, Daley TD, Darling MR (2014) Expression and localization of osteopontin, homing cell adhesion molecule/CD44, and integrin Alphavbeta3 in mucoepidermoid carcinoma and acinic cell adenocarcinoma of salivary gland origin. Oral Surg Oral Med Oral Pathol Oral Radiol 118:320–329
Ruggles N, Triantafyllou A (2013) Lysosomal activities and cellular homeostasis in epithelial tumours of salivary glands: an immunohistochemical investigation. J Pathol 231:S34
Zarbo RJ, Regezi JA, Batsakis JG (1986) S-100 Protein in salivary gland tumors: an immunohistochemical study of 129 cases. Head Neck Surg 8:268–275
Roy S, Dhingra KK, Gupta P, Khurana N, Gupta B, Meher R (2009) Acinic cell carcinoma with extensive neuroendocrine differentiation: a diagnostic challenge. Head Neck Pathol 3:163–168
Hayashi Y, Nishida T, Yoshida H, Yanagawa T, Yura Y, Sato M (1987) Immunoreactive vasoactive intestinal polypeptide in acinic cell carcinoma of the parotid gland. Cancer 60:962–968
Hayashi Y, Deguchi H, Nakahata A, Kurashima C, Hirokawa K (1990) Immunopathological study of neuropeptide expression in human salivary gland neoplasms. Pathobiology 58:212–220
Cox ML, Gourley RD, Kitabchi AE (1970) Acinic cell adenocarcinoma of the parotid with ectopic production of adrenocorticotropic hormone. Am J Med 49:529–533
Jamieson L, Taylor SM, Smith A, Bullock MJ, Davis M (2007) Metastatic acinic cell carcinoma of the parotid gland with ectopic ACTH syndrome. Otolaryngol Head Neck Surg 136:149–150
Murakami M, Ohtani I, Hojo H, Wakasa H (1992) Immunohistochemical evaluation with Ki-67: an application to salivary gland tumours. J Laryngol Otol 106:35–38
Leivo I (2006) Insights into a complex group of neoplastic disease: advances in histopathologic classification and molecular pathology of salivary gland cancer. Acta Oncol 45:662–668
Timon CI, Dardick I, Panzarella T, Patterson B, Thomas MJ, Ellis GL, Gullane PJ (1994) Acinic cell carcinoma of salivary glands. prognostic relevance of DNA flow cytometry and nucleolar organizer regions. Arch Otolaryngol Head Neck Surg 120:727–733
Nordkvist A, Roijer E, Bang G, Gustafsson H, Behrendt M, Ryd W, Thoresen S, Donath K, Stenman G (2000) Expression and mutation patterns of P53 in benign and malignant salivary gland tumors. Int J Oncol 16:477–483
Ettl T, Schwarz-Furlan S, Haubner F, Muller S, Zenk J, Gosau M, Reichert TE, Zeitler K (2012) The PI3K/AKT/MTOR signalling pathway is active in salivary gland cancer and implies different functions and prognoses depending on cell localisation. Oral Oncol 48:822–830
Suzuki S, Dobashi Y, Minato H, Tajiri R, Yoshizaki T, Ooi A (2012) EGFR and HER2-Akt-MTOR signaling pathways are activated in subgroups of salivary gland carcinomas. Virchows Arch 461:271–282
Clauditz TS, Reiff M, Gravert L, Gnoss A, Tsourlakis MC, Munscher A, Sauter G, Bokemeyer C, Knecht R, Wilczak W (2011) Human epidermal growth factor receptor 2 (HER2) in salivary gland carcinomas. Pathology 43:459–464
Clauditz TS, Gontarewicz A, Lebok P, Tsourlakis MC, Grob TJ, Munscher A, Sauter G, Bokemeyer C, Knecht R, Wilczak W (2012) Epidermal growth factor receptor (EGFR) in salivary gland carcinomas: potentials as therapeutic target. Oral Oncol 48:991–996
Jordan R, Dardick I, Lui E, Birek C (1994) Demonstration of C-ErbB-2 oncogene overexpression in salivary gland neoplasms by in situ hybridization. J Oral Pathol Med 23:226–231
Piechocki MP, Yoo GH, Dibbley SK, Amjad EH, Lonardo F (2006) Iressa induces cytostasis and augments fas-mediated apoptosis in acinic cell adenocarcinoma overexpressing HER2/Neu. Int J Cancer 119:441–454
Sams RN, Gnepp DR (2013) P63 expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol 7:64–68
Stenman G (2013) Fusion oncogenes in salivary gland tumors: molecular and clinical consequences. Head Neck Pathol 7(Suppl 1):12–19
Nasser SM, Faquin WC, Dayal Y (2003) Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. Am J Clin Pathol 119:801–806
Hamper K, Caselitz J, Arps H, Askensten U, Auer G, Seifert G (1989) The relationship between nuclear DNA content in salivary gland tumors and prognosis. Comparison of mucoepidermoid tumors and acinic cell tumors. Arch Otorhinolaryngol 246:328–332
Hamper K, Mausch HE, Caselitz J, Arps H, Berger J, Askensten U, Auer G, Seifert G (1990) Acinic cell carcinoma of the salivary glands: the prognostic relevance of DNA cytophotometry in a retrospective study of long duration (1965–1987). Oral Surg Oral Med Oral Pathol 69:68–75
El-Naggar AK, Batsakis JG, Luna MA, McLemore D, Byers RM (1990) DNA flow cytometry of acinic cell carcinomas of major salivary glands. J Laryngol Otol 104:410–416
Weiler C, Reu S, Zengel P, Kirchner T, Ihrler S (2009) Obligate basal cell component in salivary oncocytoma facilitates distinction from acinic cell carcinoma. Pathol Res Pract 205(12):838–842
Bilal H, Handra-Luca A, Bertrand JC, Fouret PJ (2003) P63 is expressed in basal and myoepithelial cells of human normal and tumor salivary gland tissues. J Histochem Cytochem 51:133–139
Bishop JA, Yonescu R, Batista DA, Westra WH, Ali SZ (2013) Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol 121:228–233
Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH (2013) Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol 44:1982–1988
Fehr A, Loning T, Stenman G (2011) Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Am J Surg Pathol 35:1600–1602
Chiosea SI, Griffith C, Assaad A, Seethala RR (2012) The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am J Surg Pathol 36:343–350
Skalova A (2013) Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol 7(Suppl 1):30–36
Mariano FV, dos Santos HT, Azanero WD, da Cunha IW, Coutinho-Camilo CM, de Almeida OP, Kowalski LP, Altemani A (2013) Mammary analogue secretory carcinoma of salivary glands is a lipid-rich tumour, and adipophilin can be valuable in its identification. Histopathology 63:558–567
Majewska H, Skalova A, Stodulski D, Klimkova A, Steiner P, Stankiewicz C, Biernat W (2015) Mammary analogue secretory carcinoma of salivary glands: a new entity associated with ETV6 gene rearrangement. Virchows Arch 466:245–254
Shah AA, Wenig BM, LeGallo RD, Mills SE, Stelow EB (2015) Morphology in conjunction with immunohistochemistry is sufficient for the diagnosis of mammary analogue secretory carcinoma. Head Neck Pathol 9:85–95
Patel KR, Solomon IH, El-Mofty SK, Lewis JS Jr, Chernock RD (2013) Mammaglobin and S-100 immunoreactivity in salivary gland carcinomas other than mammary analogue secretory carcinoma. Hum Pathol 44(11):2501–2508
Castle JT, Thompson LD, Frommelt RA, Wenig BM, Kessler HP (1999) Polymorphous low grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer 86:207–219
Schwartz LE, Begum S, Westra WH, Bishop JA (2013) GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol 7:311–315
Reis-Filho JS, Natrajan R, Vatcheva R, Lambros MB, Marchio C, Mahler-Araujo B, Paish C, Hodi Z, Eusebi V, Ellis IO (2008) Is acinic cell carcinoma a variant of secretory carcinoma? A FISH study using ETV6′split apart’ probes. Histopathology 52:840–846
Lei Y, Chiosea SI (2012) Re-evaluating historic cohort of salivary acinic cell carcinoma with new diagnostic tools. Head Neck Pathol 6:166–170
Griffith C, Seethala R, Chiosea SI (2011) Mammary analogue secretory carcinoma: a new twist to the diagnostic dilemma of zymogen granule poor acinic cell carcinoma. Virchows Arch 459:117–118
Eversole LR (1971) Histogenic classification of salivary tumors. Arch Pathol 92:433–443
Batsakis JG (1980) Salivary gland neoplasia: an outcome of modified morphogenesis and cytodifferentiation. Oral Surg Oral Med Oral Pathol 49:229–232
Gustafsson H, Carlsoo B, Henriksson R (1985) Ultrastructural morphometry and secretory behavior of acinic cell carcinoma. Cancer 55:1706–1710
Triantafyllou A, Fletcher D, Scott J (2001) Histochemical phenotypes of von Ebner’s gland of ferret and their functional implications. Histochem J 33:173–181
Dardick I, Burford-Mason AP, Garlick DS, Carney WP (1992) The pathobiology of salivary gland: II. Morphological evaluation of acinic cell carcinomas in the parotid gland of male transgenic (MMTV/v-Ha-Ras) mice as a model for human tumours. Virchows Arch A Pathol Anat Histopathol 421:105–113
Diegel CR, Cho KR, El-Naggar AK, Williams BO, Lindvall C (2010) Mammalian target of rapamycin-dependent acinar cell neoplasia after inactivation of Apc and Pten in the mouse salivary gland: implications for human acinic cell carcinoma. Cancer Res 70:9143–9152
El-Naggar AK, Abdul-Karim FW, Hurr K, Callender D, Luna MA, Batsakis JG (1998) Genetic alterations in acinic cell carcinoma of the parotid gland determined by microsatellite analysis. Cancer Genet Cytogenet 102:19–24
Stenman G (2005) Fusion oncogenes and tumor type specificity-insights from salivary gland tumors. Semin Cancer Biol 15:224–235
Nichols AC, Chan-Seng-Yue M, Yoo J, Agrawal SK, Starmans MH, Waggott D, Harding NJ, Dowthwaite SA, Palma DA, Fung K, Wehrli B, Macneil SD, Lambin P, Winquist E, Koropatnick J, Mymryk JS, Boutros PC, Barrett JW (2013) A case report and genetic characterization of a massive acinic cell carcinoma of the parotid with delayed distant metastases. Case Rep Oncol Med 2013:270362
Hsieh MS, Chou YH, Yeh SJ, Chang YL (2015) Papillary-cystic pattern is characteristic in mammary analogue secretory carcinomas but is rarely observed in acinic cell carcinomas of the salivary gland. Virchows Arch 467:145–153
Vander Poorten VLM, Balm AJM, Hilgers FJM (2002) Management of cancer of the parotid gland. Curr Opin Otolaryngol Head Neck Surg 10:134–144
Andreoli MT, Andreoli SM, Shrime MG, Devaiah AK (2012) Radiotherapy in parotid acinic cell carcinoma: does it have an impact on survival? Arch Otolaryngol Head Neck Surg 138:463–466
Spiro RH, Armstrong J, Harrison L, Geller NL, Lin SY, Strong EW (1989) Carcinoma of major salivary glands. Recent trends. Arch Otolaryngol Head Neck Surg 115:316–321
Colmenero C, Patron M, Sierra I (1991) Acinic cell carcinoma of the salivary glands. A review of 20 new cases. J Craniomaxillofac Surg 19:260–266
Eneroth CM, Jakobsson PA, Blanck C (1966) Acinic cell carcinoma of the parotid gland. Cancer 19:1761–1772
Pou AM, Myers EN (1994) Pathologic quiz case 1. Acinic cell carcinoma (acc) of the parotid gland. Arch Otolaryngol Head Neck Surg 120:1016–1019
Terhaard CH, Lubsen H, Rasch CR, Levendag PC, Kaanders HH, Tjho-Heslinga RE, van Den Ende PL, Burlage F (2005) The Role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys 61:103–111
Breen JT, Carlson ML, Link MJ, Moore EJ, Neff BA, Driscoll CL (2012) Skull base involvement by acinic cell carcinoma of the parotid gland. J Neurol Surg B Skull Base 73:371–378
Biron VL, Lentsch EJ, Gerry DR, Bewley AF (2014) Case-control analysis of survival outcomes in sinonasal acinic cell carcinoma. Int Forum Allergy Rhinol 4:507–511
Vander Poorten VLM, Balm AJM, Hilgers FJM, Tan IB, Loftus-Coll BM, Keus RB, van Leeuwen FE, Hart AAM (1999) The development of a prognostic score for patients with parotid carcinoma. Cancer 85:2057–2067
Vander Poorten V, Hart A, Vauterin T, Jeunen G, Schoenaers J, Hamoir M, Balm A, Stennert E, Guntinas-Lichius O, Delaere P (2009) Prognostic index for patients with parotid carcinoma: international external validation in a Belgian–German database. Cancer 115:540–550
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was written by members and invitees of the International Head and Neck Scientific Group (http://www.IHNSG.com).
Rights and permissions
About this article
Cite this article
Vander Poorten, V., Triantafyllou, A., Thompson, L.D.R. et al. Salivary acinic cell carcinoma: reappraisal and update. Eur Arch Otorhinolaryngol 273, 3511–3531 (2016). https://doi.org/10.1007/s00405-015-3855-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-015-3855-7