Abstract
Data indicate a better prognosis for human papillomavirus (HPV)-associated head and neck squamous cell carcinoma (HNSCC). HPV and p16 detection are established markers for HPV-related HNSCC. Both are accepted as survival-independent predictors. Previous studies investigating the survival in HNSCC patients depending on HPV+/− and p16+/− status consistently found discordant results with p16−/HPV+ and p16+/HPV−. However, no meta-analysis regarding the survival according to combined HPV/p16 status has been performed yet. The objective of this study was to discriminate the impact of combined HPV+/− and p16+/− status on survival. Data sources were identification and review of publications assessing survival of the distinct subgroups with both p16 and HPV investigated in HNSCC until February, 2015. A meta-analysis was performed to classify survival and clinical outcomes. 18 out of 397 articles (4424 patients) were eligible for the meta-analysis. The percent proportion of the subgroups was 25 % for HPV+/p16+, 61.2 % for HPV−/p16−, 7.1 % for HPV−/p16+ and 6.8 % for HPV+/P16−. The meta-analysis showed a significantly improved 5-year overall survival (OS), 5-year disease-free survival and their corresponding hazard ratio for HPV+/p16+ HNSCC in comparison to HPV−/p16−, HPV+/p16− and HPV−/p16+. The 5-year OS of the HPV−/p16+ subgroup was intermediate while HPV+/p16− and HPV−/p16− HNSCC had the shortest survival. With current therapeutic strategies, survival of patients with HNSCC is better if associated with HPV+/p16+ or HPV−/p16+. Clinical trials are needed to confirm the distinct survival pattern and to investigate possible differences in survival for HPV+/p16− and HPV−/p16+ HNSCC. To further differentiate p16+ HNSCC, HPV testing may be advisable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy worldwide with 350,000 deaths per year [1]. Despite modern treatment strategies, local recurrence rates, metastases, and overall survival (OS) have not changed much in the last 30 years [21, 31, 51]. In recent years it has been accepted that HNSCC etiologically associated with excessive alcohol and tobacco consumption is characterized by specific p53 mutations. This has to be distinguished from HNSCC etiologically associated with persistent infection with the oncogenic high-risk human papillomaviruses (HR-HPV). These generally contain wild-type p53 [24] as well as an increased expression of p16, a marker for the oncogenic activity of HR-HPV [24, 33]. Proteins derived from the viral oncogenes HPV-E6 and HPV-E7 form complexes with tumor suppressor gene products leading to p53 degradation and pRb inactivation, respectively [70, 81]. This is followed by a suppression of apoptosis and initiates a transition to active cell cycling. The aforementioned oncogenes also prevent a physiological immune response to virus-infected tumor cells preventing their elimination [5, 22]. Overall, HR-HPV-associated HNSCC amount currently to 25 % and in oropharyngeal HNSCC (OSCC) up to 70 % [23, 44]. The incidence of HPV-associated HNSCC is expected to rise further [42]. Evidence-based clinical guidelines recommend HPV testing in HNSCC, especially for those arising in the oropharynx. HPV status may provide prognostic information and in future, it may also guide specific treatment decisions [17, 19]. However, a standardized procedure for HPV testing remains to be established [6, 9, 38]. The HPV protein E7 leads to pRb degradation which in turn overexpresses p16. Consequently, overexpressed p16 has been used as a surrogate marker for transcriptionally active HPV in OSCC [50, 79]. All HPV-specific tests, including p16-IHC, show a strong correlation with patient survival [e.g., presence of HPV-RNA by RT-PCR, HPV-RNA by in situ hybridization (ISH), and p16-IHC] [6, 50, 63, 67, 79]. And the expression of both markers correlates significantly [12]. So far, all major multivariate survival analyses have demonstrated that a positive p16-IHC status, independently, correlated with better survival [6, 50, 64, 67, 79]. Thus, HPV-DNA-positivity and p16 overexpression represent independent prognostic risk factors for improved survival [77].
Robinson et al. found by HPV-DNA testing in a study including 496 patients that only 5 % of cases were p16+/HPV− and that 8 % were p16−/HPV+ [60]. Other, studies confirmed this finding by HPV E6/E7 mRNA testing and defined the range for p16+ tumors to be HPV-RNA−between 1 and 7 % and of p16− tumors to be HPV-RNA+ between 2 and 7 % [50, 63, 65]. At this time, it remains unclear whether these consistently made observations have an underlying and so far unknown biological cause or represent an artifact due to insufficient sensitivity and specificity of existing HPV- and p16-testing.
Objectives
Despite the relatively low rate of discrepant cases (p16−/HPV+ or p16+/HPV−), the aim of the present study was to perform a meta-analysis on the survival of these distinct groups in comparison to the p16+/HPV+ and p16−/HPV− groups.
Methods
Literature search strategy and selection criteria
We searched for published literature evaluating the survival of subgroups according to the detection of both HPV and p16 markers three databases: PubMed (http://www.ncbi.nlm.nih.gov/pubmed), EMBASE (“http://www.embase.com/”) and Wanfang (http://www.wanfangdata.com.cn) up to February 14th, 2015. The following search terms were used “HPV, p16, head neck”. Additionally, we checked references cited in original or review articles that were not retrieved from the database by the initial literature search. The manuscripts were screened and all studies on HNSCC patients investigating survival rates according to the tumors’ p16 and HPV status were included. Exclusion criteria were missing information on patient survival and investigation of only one marker (HPV or p16), primary cancer different from HNSCC (nasopharyngeal carcinoma, skin cancer, pre-cancer), cell culture or animal models, and reviews or case reports. In a more detailed second round of selection, we excluded studies with duplicate patient data from the same or similar population according to authors’ names and institution. In these instances, for further analysis the more recent study or the study with a larger patient number was selected. Additionally, we excluded studies with insufficient survival data for this meta-analysis. Finally, this meta-analysis includes studies with the following criteria: (1) in numbers the portion of the subgroups HPV+/p16+ versus HPV−/p16− versus HPV+/p16− versus HPV−/p16+ in HNSCC patients; (2) in numbers survival data of these subgroups [hazard ratio (HR); overall survival (OS); disease-free survival (DFS)] or Kaplan–Meier curves of the subgroups of OS or DFS.
Data extraction
From all eligible publications, the relevant data were extracted by two of the authors (A.C. and A.E.A.) independently following to the inclusion criteria defined for this study. Discrepancies in findings were decided by a reanalysis of the study followed by a final decision by both authors. All relevant information from the manuscript, tables and figures of the incorporated studies, such as author information, date of publication, time frame of the study, country, tumor stage and localization, number of patients, study design, alcohol and tobacco consumption, number of HPV positive and negative patients, number of patients included in the subgroups HPV+/p16+, HPV−/p16−, HPV+/p16− or HPV−/p16+, HPV subtypes, HR-status, 5-year OS or DFS of the subgroups, p16 and HPV detection method were retrieved. In articles where the OS or DFS was displayed as Kaplan–Meier plot, the software GraphClick (Version 3.0.2, Arizona Software 2010, http://www.arizona-software.ch/graphclick) was used to compute the data.
Statistical analysis
For statistical analysis, we used the relative risk (RR) to evaluate OS and DFS of all subgroups in correlation to the HPV+/p16+ and HPV−/p16− groups. Summary RR estimates and 95 % confidence intervals (CI) were calculated using maximum-likelihood methods for linear mixed models. To assess study heterogeneity a Chi-squared-based Q test was used. A resulting P value above 0.05 indicated an absence of heterogeneity between the studies. In the meta-analysis, the I 2 index was used to search for any existing heterogeneity, which was estimated as percentage from 0 to 100 %. First, a fixed-effects model (Mantel–Haenszel method and Chi-squared test) was fitted to the data. If the heterogeneity was significant the random-effects model (DerSimonian–Liard method) was used. Depending on the extracted data of the individual publications, we studied the RR of the 5-year OS and DFS of all subgroups in correlation to the HPV+/p16+- and HPV−/p16− groups. The same analyses were performed if the HR was described in the studies. To compare all included studies a forest plot was used.
Analysis of publication bias was determined by using a funnel plot. All statistical analyses were performed using the computing environment R Version 3.1.0 (R Core Team [56]).
Results
Description of the included studies
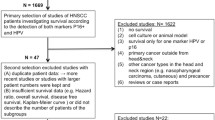
A total of 18 out of 397 publications met the criteria for this analysis [11, 15, 25, 26, 29, 30, 34, 36, 43, 47, 48, 58, 62, 68, 69, 75, 80, 82] (Fig. 1; Table 1). The total number of patients included in the studies was 4424 (ranging from 34 to 841 patients per study). However, not for all of these patients the HPV/p16-status was complete, so finally the data of 2811 study patients (ranging from 34 to 723 patients per study) were analyzed in this meta-analysis. The main characteristics of the eligible studies are summarized in Tables 1, 2 and 3. In 14 articles patients with UICC tumor stage I–IV were investigated. In 8 studies OSCC and in 10 HNSCC was investigated (Table 1). HPV detection after preceding polymerase chain reaction (PCR) and in situ hybridization (ISH) without earlier PCR were used as HPV detection methods in 15 and 3 studies, respectively. Table 2 indicates the number of patients belonging to the different subgroups depending on the HPV and p16-status. The proportion of the subgroups was estimated from the 16 studies, which explicitly indicated the patient number [11, 15, 25, 26, 34, 36, 43, 47, 48, 58, 62, 68, 69, 75, 80, 82]. The subgroup of HPV+/p16+ was 25 %, of HPV−/p16− 61.2 %, of HPV−/p16+ 7.1 % and of HPV+/p16− 6.8 %. Table 3 summarizes the studies investigating correlations between HPV status and clinicopathological characteristics like tumor size, lymph node involvement, localization in the OSCC, smoking, etc. 7 studies were performed in Europe, 7 studies in the United States of America (USA) and 4 in Asia.
5-year OS of the HPV/p16 subgroups
Thirteen studies determined 5-year OS of all distinct HPV/p16 subgroups. For the investigation of the 5-year OS, 12 studies (1961 patients) contained suitable data for performing a meta-analysis of HPV+/p16+ and HPV−/p16− patients. The forest plot of this meta-analysis is shown in Fig. 2a and indicates that HPV+/p16+ is associated with improved OS (fixed-effects model; RR of 2.45; 95 % CI 2.07–2.89; P = 0.8721).
Meta-analysis for adjusted relative risk (RR) of the 5-year overall survival (OS) compared to the HPV+/p16+ subgroup. Forest plot of RR among included studies for the 5-year OS of the HPV+/p16+ subgroup compared to (a) HPV−/p16−, (b) HPV−/p16+ and (c) HPV+/p16−. Combined RR was calculated by a random mode.
Eight studies (511 patients) included data for the 5-year OS of HPV+/p16+ and HPV−/p16+ patients and 6 studies (373 patients) of HPV+/p16+ and HPV+/p16− patients. The forest plot of these meta-analyses is shown in Fig. 2b, c and indicates that HPV+/p16+ has an improved OS compared with both the HPV−/p16+ subgroup (fixed-effects model; RR of 1.87; 95 % CI 1.40–2.51; P = 0.6343) and the HPV+/p16− subgroup (fixed-effects model; RR of 2.53; 95 % CI 1.96–3.26; P = 0.075).
Eight studies (1465 patients) included data for the 5-year OS of HPV−/p16− and HPV−/p16+ patients and 4 studies (421 patients) of HPV−/p16− and HPV+/p16− patients. The forest plot of the meta-analysis of HPV−/p16− and HPV−/p16+ is shown in Fig. 3a and indicates that the OS of HPV−/p16− does not significantly differ from the OS in the HPV+/p16− subgroup (fixed-effects model; RR of 0.97; 95 % CI 0.81–1.16; P = 0.8222). However, the OS in the HPV−/p16− subgroup was significantly lower compared to the HPV−/p16+ (fixed-effects model; RR of 0.82; 95 % CI 0.67–0.99; P = 0.5043; Fig. 3b).
Meta-analysis for adjusted relative risk (RR) of the 5-year overall survival (OS) compared to the HPV−/p16− subgroup. Forest plot of RR among included studies for the 5-year OS of the HPV−/p16− subgroup compared to (a) HPV−/p16+ and (b) HPV+/p16−. (c) Forest plot of RR compares HPV−/p16+ and HPV+/p16−. Combined RR was calculated by a random mode.
Furthermore, 4 studies (149 patients) included data of HPV+/p16− and HPV−/p16+ patients for 5-year OS (Fig. 3c). The subgroup of HPV+/p16− had a reduced OS compared with the subgroup of HPV−/p16+ (fixed-effects model; RR of 0.56, 95 % CI 0.42–0.73, P = 0.0809).
HR of the OS of the HPV/p16 subgroups
The HR for the OS could be determined from data included in 10 studies. Four studies used HPV+/p16+ and 6 used HPV−/p16− as a reference marker. The results of the individual meta-analyses are summarized in Table 4. The HRs for the OS of the HPV+/p16+ subgroup were significantly increased compared to the HPV−/p16− subgroup regardless whether HPV+/p16+ or HPV−/p16− was used as reference value. The further meta-analyses included only 2–3 studies. Thus they are more limited in their value for general conclusions. The HRs for the OS of the HPV+/p16+ subgroup was significantly increased compared to the HPV−/p16+ subgroup; however, it did not significantly differ from the HPV+/p16− subgroup. The HRs for the OS of the HPV−/p16− subgroup did not significantly differ from the HPV−/p16+ and HPV+/p16− subgroup.
5-year DFS of the distinct HPV/p16 subgroups
5-year DFS was investigated in 6 studies. The results of the individual meta-analyses of the different subgroups in relation to the individual HPV and p16-status are summarized in Table 5. Six studies, including 1430 patients, showed suitable data to perform a meta-analysis on the 5-year DFS of HPV+/p16+ and HPV−/p16− patients. The HPV+/p16+ subgroup was associated with a significantly improved DFS (fixed-effects model; RR 2.06; 95 % CI 1.73–2.44; P = 0.1116). The meta-analyses included 3–4 studies and are summarized in Table 5. However, the studies did not have sufficient data to perform a meta-analysis of the subgroups HPV+/p16− and HPV−/p16+. The HPV+/p16+ subgroup was associated with a significantly improved DFS compared to both the HPV−/p16+ DFS (fixed-effects model; RR 1.4; 95 % CI 1.02–1.92; P = 0.2697) and HPV+/p16−subgroup DFS (random-effects model; RR 2.52; 95 % CI 1.11–5.74; P < 0.001). The HPV−/p16− subgroup was associated with an improved DFS compared to the HPV+/p16− DFS (random-effects model; RR 1.69; 95 % CI 1.06–2.69; P = 0.0034).
The HR for the DFS was investigated in 6 studies. The HPV+/p16+ subgroup was associated with a significantly improved DFS (random-effects model; RR 2.63; 95 % CI 2.60–2.67; P < 0.001).
Sensitivity analysis
We performed a sensitivity analysis in order to test for a possible bias resulting from the low numbers of available eligible publications. Therefore, we divided the meta-analysis of the 5-year OS of the HPV+/p16+ and HPV−/p16− subgroups according to the continent where the study had been performed. These sub-meta-analyses from the US, Europe and Asia showed comparable results to the whole meta-analyses with the complete data set of all international studies (Fig. 2a). Due to non-significant heterogeneity (P > 0.05), the fixed-effects model was used in all sub-meta-analyses (data not shown), indicating that our results were statistically robust. The RR and CI were essentially not altered compared with the whole meta-analyses.
Finally, to test for a possible bias resulting from differences of the HPV detection methods used in the included studies, a sensitivity analysis was performed. We divided the meta-analysis of the 5-year OS of the HPV+/p16+ and HPV−/p16− subgroups according to the HPV detection methods into two groups: one group using PCR and one group using ISH without PCR. These two meta-analyses showed comparable results to the meta-analyses where all data were included (Fig. 2a). As before due to non-significant heterogeneity (P > 0.05), the fixed-effects model was used (data not shown), indicating that our results were statistically robust. The RR and CI were essentially not altered compared with the meta-analyses of all data.
Publication bias
The shape of the funnel plots did not reveal obvious evidence of asymmetry.
Discussion
Recent increases in incidence of HNSCC and survival of HNSCC patients, especially in countries with decreasing tobacco abuse, have been attributed to HPV-associated HNSCC. It is therefore of clinical relevance to increase current knowledge about (a) the incidence of HPV-associated HNSCC and its surrogate markers; (b) the clinical course of the etiologically distinct HNSCC-types and (c) whether further subdivision of HNSCC according to their specific HPV/p16 status has any clinical relevance. The aim of such a subdivision would be to ultimately adapt therapy intensity to a specific HNSCC-subtype.
The expression of both markers p16-positive IHC and HPV-DNA positivity correlates significantly in HNSCC [12]. There is, however, a certain discrepancy rate of approximately 10 %. In these cases, HNSCC with the marker-combinations HPV+/p16− or HPV−/16+ are found [28, 38, 50, 63, 65]. In the present study, we therefore performed a meta-analysis in HNSCC to determine if the survival of these distinct subgroups is different from the more frequent HPV+/p16+ and HPV−/p16− groups. To this date it is unclear if these discrepancies are real and have an as yet undefined biological explanation or if they are merely an artifact due to the limitations in detection specificity/sensitivity. To approach this question from an epidemiological perspective, we set out to investigate if there were differences in the clinical courses of the different patient groups.
A number of meta-analyses on HPV-associated HNSCC have been conducted so far, yet none has investigated the survival of patients by combining information about HPV status and p16 [12, 35, 54, 71]. Most of them showed the survival benefit of either HPV or p16 individually in two separate meta-analyses [55]. For this study, we searched for published studies evaluating the survival of subgroups according to the detection of both markers, i.e., HPV and p16. We did not subdivide the studies according to the percentage of p16 positive cells in order to increase the number of studies of the meta-analyses [37]. Ultimately, 18 studies out of 397 publications met the inclusion criteria, i.e., reporting the numbers of patients within the subgroups of HPV+/p16+, HPV−/p16−, HPV+/p16− and HPV−/p16+, and their HR, OS or Kaplan–Meier curves of OS or DFS, respectively [11, 15, 25, 26, 29, 30, 34, 36, 43, 47, 48, 58, 62, 68, 69, 75, 80, 82] (Fig. 1). 14 studies investigated patients with UICC tumor stages I–IV. 8 studies investigated OSCC only and 10 investigated HNSCC. Despite the higher prevalence of HPV and p16 in OSCC [74], we included all HNSCC locations in this meta-analysis to increase the total number of patients and studies and to allow for a separate evaluation of the discrepant cases (HPV+/p16− and HPV−/p16+). Therefore, the number of studies included in this analysis is smaller than in other previously published meta-analyses with different inclusion criteria that investigated the markers independently [12, 35, 71].
Seven studies were performed in Europe, 7 in the US and 4 in Asia. Sensitivity analysis showed that the results of the 5-year OS were comparable in all continents. Therefore, a geographic differentiation of the study origin was not necessary. However, in future, as more data are available, such geographic distinctions may become relevant, since tobacco consumption and the incidence of HPV infection show differences between countries and continents [4, 10, 61]. Cofactors such as smoking behavior may impact the prevalence and presence of the two markers HPV and p16, but not the survival of the distinct subgroups [8, 72]. In 50 % of the studies the detection of HPV was associated with non-smoking. Smoking, however, has been shown to be an independent risk factor of reduced treatment efficiency and OS in HPV-related carcinomas [2, 20, 45]. Efforts to investigate prospectively relationships between tobacco consumption, HPV and p16 status and survival have been initiated at our institution [72]; however, data for the distinct subgroups are not yet available.
The method of HPV detection is critical for accurate assessment of the HPV status. HPV detection by PCR and by ISH only without PCR were used as HPV detection methods in 15 and 3 studies, respectively. Sensitivity analysis showed that the results of the 5-year OS were comparable using both HPV detection methods. In our meta-analysis, the subgroup categories of HPV+/p16+ patients included 25 %, of HPV−/p16− 61.2 %, of HPV−/p16+ 7.1 % and of HPV+/p16− 6.8 % patients, which is in line with earlier studies focussing on the number of discrepant cases without investigating the survival [3, 28, 38]. HPV testing is not yet standardized [6, 38]. Recently, Westra et al. [78] suggested one method for HPV detection. All HPV-specific tests including surrogate p16-IHC showed a strong and independent correlation with patient survival (e.g., HPV-RNA detection by RT-PCR, RNA detection by ISH, and p16-IHC) [6, 50, 63, 64, 67, 77, 79]. The actual prevalence of HPV in HPV-associated HNSCC depends on the sensitivity of the detection method. HPV testing by PCR has a slightly increased sensitivity over ISH [71]. The lack of data on the exact specificity of the PCR prevents a precise indication of the number of false-positive patients. HPV+/p16− cases are more similar to HPV−/p16− in terms of their genetic profiling, suggesting that HPV may be an innocent bystander in these samples and not directly involved in the process of the carcinogenesis [59, 76]. Expression of E6 and E7 mRNA is highly associated with p16 expression [27, 32, 33, 40, 53, 79]. However, the quality of results using mRNA detection is still controversial [14]. The number of studies investigating the survival of p16-positive HNSCC and E6/E7 oncoprotein detection is not sufficient to perform a meta-analysis. Recently, E6/E7-specific antibodies have been developed that facilitate immunohistochemisty for detection of transforming infection. Those have not been used in conjunction with clinical studies. Also the measurement of activity of the viral oncoproteins has been used in experimental studies. Such methods, however, are not suitable for testing of clinical study materials yet. Therefore, the IHC for p16 may be a reasonable surrogate for detection of transcriptionally active HPV infection [67] if combined with HPV-DNA testing. In HPV− cases p53 mutations are common [7]. However, HPV-independent pathways of oncogenesis can also lead to an increased expression of p16. Moreover, p16 has been identified to have a distinct function in cellular transformation and is not just a surrogate marker. Knock out of p16 in HPV E7 expressing cells can lead to induction of apoptosis showing a physiological role in transformation despite its original role in the process of senescence [41, 49].
The specificity of the p16 IHC is only 79 % [3, 67]. Selecting for HPV16-DNA positive cases only, one study found a 93 % correlation between p16 expression and HPV status [66]. Some of the discordant results could be explained by the presence of HPV types other than type 16. HPV16 accounts for 78.6–100 % of HPV+ OSCC cases [35]. However, there still remains a discordant group of p16-negative cases in the HPV16+ compartment [3, 50, 63, 65].
Patients with HNSCC that test HPV+/p16+ have a significantly improved survival. This meta-analysis was able to demonstrate this for the 5-year OS, 5-year DFS and their corresponding HR. The same superiority in survival for HPV+/p16+ was demonstrated in comparison with HPV+/p16− and HPV−/p16+ patients for the 5-year OS and DFS. The meta-analyses investigating OS of HPV−/p16− HNSCC compared to HPV+/p16− and HPV−/p16+ showed a significantly lower survival compared to the HPV−/p16+ subgroup; however, the 5-year OS was comparable with the HPV+/p16− subgroup (Fig. 3). The meta-analysis comparing HPV−/p16+ and HPV+/p16− showed that the 5-year OS of the subgroup of HPV−/p16+ was increased compared with HPV+/p16− (Fig. 3c). The results confirm that the different HPV/p16 subgroups differ in their prognoses. The meta-analysis also proved that p16 can be used as a surrogate marker for HPV-related HNSCC. However, for a precise statement of the prognosis, each possible combination of p16/HPV has to be regarded as a separate group concerning prognosis. Thus, the detection of p16+ HNSCC should be followed by a specific HPV detection method, especially if innovative therapy strategies are taken into account for cancer treatment [39]. Therefore, patient samples with detection of one or both markers for HPV or p16 should not be summarized into one single group [13, 29, 30]. The data from other groups and our own indicated that HPV-related HNSCC have a distinct biology [52], and are more responsive to treatment by radio- and chemotherapy [16, 17, 19].
The clinicopathological factors like tumor size, lymph node involvement, localization are comparable with those analyzed in other meta-analyses (Table 3) [12].
There are several limitations of our study: (a) the study is based on data extracted from the published literature and not from individual patient data. (b) Although a relatively high total number of patients were included in the meta-analysis, the number of the studies containing the data that were used to calculate specific associations was relatively small. A limitation that may only be overcome with access to primary data from all included publications and when further studies including more patients where HPV and p16 are determined simultaneously became available. (c) It is still necessary to determine the best method for tumor sampling and subsequent HPV and p16 detection. This will also help validating the role of p16-overexpression as surrogate marker for HPV-related HNSCC, as it is now in clinical practice of some centers. According to data form this study this practice has to be questioned. For now, HPV should be detected on a RNA or DNA level to further increase the sensitivity and to provide information about active transcription. (d) When selecting eligible studies for this analysis, an overlap of the same patient series included in multiple reports was observed in some instances. In these cases only the largest was included in our analysis.
In spite of the limitations inherent to this type of meta-analysis, our results are in line with published literature and our current understanding of the biology of HPV-related HNSCC. To advance current treatments, data from large randomized prospective clinical trials are needed to determine the best treatment for patients based on the tumor’s HPV- and p16-status. In our view, HNSCC with HPV+/p16−- and HPV−/p16+-status are of particular interest to clarify the prognosis and response to therapy and the necessity to determine the HPV status as discussed above in clinical routine. Also further confirmation of improved responses to current treatment modalities of patients with HPV+/p16+ HNSCC may result in curative intent therapy regimen with a reduction in radiation- or chemotherapy dosage to reduce undesired side-effects [57]. Second, given the etiologic involvement of the HPV oncoproteins E6 and E7 during carcinogenesis of these tumors [73], it is also conceivable that intervention with drugs targeting E6 or E7 proteins or inhibiting virus-induced dysregulation (e.g., inactivation of p53, etc.) may enhance sensitivity of these tumors to cytotoxic drugs. HPV-16 is the most prevalent genotype in HPV-related HNSCC which may be prevented by a today’s HPV-vaccination if applied in both sexes [18, 46].
Conclusions
In conclusion, survival from HNSCC is improved in patients with HPV+/p16+-status, intermediate in patients of HPV−/p16+ status and most limited in patients with the combinations of HPV−/p16− or HPV+/p16−. Larger trials are mandatory to investigate the survival of patients including those with HPV+/p16− and HPV−/p16+ HNSCC more closely and should aim to adapt future therapeutic regimen according to the HPV/p16-status. The data indicate, that p16+ HNSCC should be subdifferentiated by HPV-typing to further identify HPV-associated HNSCC. Last, laboratory studies should aim to elucidate the underlying biological cause of the distinct HPV/p16 pattern.
References
Albers AE, Chen C, Koberle B et al (2012) Stem cells in squamous head and neck cancer. Crit Rev Oncol Hematol 81:224–240
Ang KK, Harris J, Wheeler R et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35
Begum S, Cao D, Gillison M et al (2005) Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res 11:5694–5699
Bossi P, Orlandi E, Miceli R et al (2014) Treatment-related outcome of oropharyngeal cancer patients differentiated by HPV dictated risk profile: a tertiary cancer centre series analysis. Ann Oncol 25:694–699
Bottley G, Watherston OG, Hiew YL et al (2008) High-risk human papillomavirus E7 expression reduces cell-surface MHC class I molecules and increases susceptibility to natural killer cells. Oncogene 27:1794–1799
Braakhuis BJ, Brakenhoff RH, Meijer CJ et al (2009) Human papilloma virus in head and neck cancer: the need for a standardised assay to assess the full clinical importance. Eur J Cancer 45:2935–2939
Braakhuis BJ, Rietbergen MM, Buijze M et al (2014) TP53 mutation and human papilloma virus status of oral squamous cell carcinomas in young adult patients. Oral Dis 20:602–608
Cerezo L, Lopez C, De La Torre A et al (2014) Incidence of human papillomavirus-related oropharyngeal cancer and outcomes after chemoradiation in a population of heavy smokers. Head Neck 36:782–786
Chai RC, Lambie D, Verma M et al (2015) Current trends in the etiology and diagnosis of HPV-related head and neck cancers. Cancer Med
Chaturvedi AK, Engels EA, Pfeiffer RM et al (2011) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294–4301
Chung CH, Zhang Q, Kong CS, Harris J, Fertig EJ, Harari PM, Wang D, Redmond KP, Shenouda G, Trotti A, Raben D, Gillison ML, Jordan RC, Le QT (2014) p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol 32(35):3930–3938. doi:10.1200/JCO.2013.54.5228
Dayyani F, Etzel CJ, Liu M et al (2010) Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol 2:15
Deng Z, Hasegawa M, Aoki K et al (2014) A comprehensive evaluation of human papillomavirus positive status and p16INK4a overexpression as a prognostic biomarker in head and neck squamous cell carcinoma. Int J Oncol 45:67–76
Dreyer JH, Hauck F, Oliveira-Silva M et al (2013) Detection of HPV infection in head and neck squamous cell carcinoma: a practical proposal. Virchows Arch 462:381–389
Evans M, Newcombe R, Fiander A, Powell J, Rolles M, Thavaraj S, Robinson M, Powell N (2013) Human Papillomavirus-associated oropharyngeal cancer: an observational study of diagnosis, prevalence and prognosis in a UK population. BMC Cancer 13:220. doi:10.1186/1471-2407-13-220
Fakhry C, Westra WH, Li S et al (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261–269
Flavill E, Fang YV, Miles B et al (2014) Induction chemotherapy followed by concurrent chemoradiotherapy for advanced stage oropharyngeal squamous cell carcinoma with HPV and P16 testing. Ann Otol Rhinol Laryngol 123:365–373
Garland SM, Hernandez-Avila M, Wheeler CM et al (2007) Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 356:1928–1943
Geiger JL, Lazim AF, Walsh FJ et al (2014) Adjuvant chemoradiation therapy with high-dose versus weekly cisplatin for resected, locally-advanced HPV/p16-positive and negative head and neck squamous cell carcinoma. Oral Oncol 50:311–318
Granata R, Miceli R, Orlandi E et al (2012) Tumor stage, human papillomavirus and smoking status affect the survival of patients with oropharyngeal cancer: an Italian validation study. Ann Oncol 23:1832–1837
Greenlee RT, Hill-Harmon MB, Murray T et al (2001) Cancer statistics, 2001. CA Cancer J Clin 51:15–36
Guirat-Dhouib N, Baccar Y, Mustapha IB et al (2012) Oral HPV infection and MHC class II deficiency (A study of two cases with atypical outcome). Clin Mol Allergy CMA 10:6
Hammarstedt L, Lindquist D, Dahlstrand H et al (2006) Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer 119:2620–2623
Haraf DJ, Nodzenski E, Brachman D et al (1996) Human papilloma virus and p53 in head and neck cancer: clinical correlates and survival. Clin Cancer Res 2:755–762
Heath S, Willis V, Allan K, Purdie K, Harwood C, Shields P, Simcock R, Williams T, Gilbert DC (2012) Clinically significant human papilloma virus in squamous cell carcinoma of the head and neck in UK practice. Clin Oncol 24(1):e18–e23. doi:10.1016/j.clon.2011.05.007
Heiduschka G, Grah A, Oberndorfer F, Kadletz L, Altorjai G, Kornek G, Wrba F, Thurnher D, Selzer E (2014) Improved survival in HPV/p16-positive oropharyngeal cancer patients treated with postoperative radiotherapy. Strahlenther Onkol. doi:10.1007/s00066-014-0753-7
Hoffmann M, Ihloff AS, Gorogh T et al (2010) p16(INK4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer 127:1595–1602
Hoffmann M, Tribius S, Quabius ES et al (2012) HPV DNA, E6*I-mRNA expression and p16INK4A immunohistochemistry in head and neck cancer—how valid is p16INK4A as surrogate marker? Cancer Lett 323:88–96
Holzinger D, Schmitt M, Dyckhoff G, Benner A, Pawlita M, Bosch FX (2012) Viral RNA patterns and high viral load reliably define oropharynx carcinomas with active HPV16 involvement. Cancer Res 72(19):4993–5003. doi:10.1158/0008-5472.CAN-11-3934
Huang H, Zhang B, Chen W, Zou SM, Xu ZG (2013) Relationship between HPV-DNA status and p16 protein expression in oropharyngeal squamous cell carcinoma and their clinical significance. Zhonghua Zhong Liu Za Zhi 35(9):684–688
Jones AS, Goodyear PW, Ghosh S et al (2011) Extensive neck node metastases (N3) in head and neck squamous carcinoma: is radical treatment warranted? Otolaryngol Head Neck Surg 144:29–35
Jung AC, Briolat J, Millon R et al (2010) Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer 126:1882–1894
Klussmann JP, Gultekin E, Weissenborn SJ et al (2003) Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 162:747–753
Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, Pourmand N, Le QT (2009) The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 74(2):553–561. doi:10.1016/j.ijrobp.2009.02.015
Kreimer AR, Clifford GM, Boyle P et al (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14:467–475
Liang C, Marsit CJ, McClean MD, Nelson HH, Christensen BC, Haddad RI, Clark JR, Wein RO, Grillone GA, Houseman EA, Halec G, Waterboer T, Pawlita M, Krane JF, Kelsey KT (2012) Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res 72(19):5004–5013. doi:10.1158/0008-5472.CAN-11-3277
Lin J, Albers AE, Qin J et al (2014) Prognostic significance of overexpressed p16INK4a in patients with cervical cancer: a meta-analysis. PLoS One 9:e106384
Marur S, D’souza G, Westra WH et al (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11:781–789
Masterson L, Moualed D, Liu ZW et al (2014) De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: a systematic review and meta-analysis of current clinical trials. Eur J Cancer 50:2636–2648
Mclaughlin-Drubin ME, Crum CP, Munger K (2011) Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc Natl Acad Sci USA 108:2130–2135
Mclaughlin-Drubin ME, Park D, Munger K (2013) Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc Natl Acad Sci USA 110:16175–16180
Mehanna H, Beech T, Nicholson T et al (2013) Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer–systematic review and meta-analysis of trends by time and region. Head Neck 35:747–755
Melkane AE, Auperin A, Saulnier P, Lacroix L, Vielh P, Casiraghi O, Msakni I, Drusch F, Temam S (2014) Human papillomavirus prevalence and prognostic implication in oropharyngeal squamous cell carcinomas. Head Neck 36(2):257–265. doi:10.1002/hed.23302
Ndiaye C, Mena M, Alemany L et al (2014) HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 15:1319–1331
O’sullivan B, Huang SH, Siu LL et al (2013) Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol 31:543–550
Paavonen J, Jenkins D, Bosch FX et al (2007) Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 369:2161–2170
Park K, Cho KJ, Lee M, Yoon DH, Kim J, Kim SY, Nam SY, Choi SH, Roh JL, Han MW, Lee SW, Song SY, Back JH, Kim SB (2013) p16 immunohistochemistry alone is a better prognosticator in tonsil cancer than human papillomavirus in situ hybridization with or without p16 immunohistochemistry. Acta Otolaryngol 133(3):297–304. doi:10.3109/00016489.2012.741327
Park WS, Ryu J, Cho KH, Choi MK, Moon SH, Yun T, Chun BS, Lee GK, Ahn HJ, Lee JH, Vermeer P, Jung YS (2012) Human papillomavirus in oropharyngeal squamous cell carcinomas in Korea: use of G1 cycle markers as new prognosticators. Head Neck 34(10):1408–1417. doi:10.1002/hed.21939
Pauck A, Lener B, Hoell M et al (2014) Depletion of the cdk inhibitor p16INK4a differentially affects proliferation of established cervical carcinoma cells. J Virol 88:5256–5262
Perrone F, Gloghini A, Cortelazzi B et al (2011) Isolating p16-positive/HPV-negative oropharyngeal cancer: an effort worth making. Am J Surg Pathol 35:774–777 (author reply 777–778)
Pignon JP, Le Maitre A, Maillard E et al (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4–14
Qian X, Wagner S, Ma C et al (2014) Prognostic significance of ALDH1A1-positive cancer stem cells in patients with locally advanced, metastasized head and neck squamous cell carcinoma. J Cancer Res Clin Oncol 140:1151–1158
Quabius ES, Haag J, Kuhnel A et al (2015) Geographical and anatomical influences on human papillomavirus prevalence diversity in head and neck squamous cell carcinoma in Germany. Int J Oncol 46:414–422
Ragin CC, Taioli E (2007) Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 121:1813–1820
Rainsbury JW, Ahmed W, Williams HK et al (2013) Prognostic biomarkers of survival in oropharyngeal squamous cell carcinoma: systematic review and meta-analysis. Head Neck 35:1048–1055
Rcoreteam (2014) R Statistical Software. A language and environment for statistical computing. R Foundation for Statistical Computing. R Version 3.1.0. In: Software RS (ed)URL, Vienna, Austria
Rieckmann T, Tribius S, Grob TJ et al (2013) HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol 107:242–246
Rietbergen MM, Brakenhoff RH, Bloemena E, Witte BI, Snijders PJ, Heideman DA, Boon D, Koljenovic S, Baatenburg-de Jong RJ, Leemans CR (2013) Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment de-escalation trials. Ann Oncol 24(11):2740–2745. doi:10.1093/annonc/mdt319
Rietbergen MM, Snijders PJ, Beekzada D et al (2014) Molecular characterization of p16-immunopositive but HPV DNA-negative oropharyngeal carcinomas. Int J Cancer 134:2366–2372
Robinson M, Sloan P, Shaw R (2010) Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol 46:492–496
Rodrigo JP, Heideman DA, Garcia-Pedrero JM et al (2014) Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990–2009). Int J Cancer 134:487–492
Salazar CR, Anayannis N, Smith RV, Wang Y, Haigentz M Jr, Garg M, Schiff BA, Kawachi N, Elman J, Belbin TJ, Prystowsky MB, Burk RD, Schlecht NF (2014) Combined P16 and human papillomavirus testing predicts head and neck cancer survival. Int J Cancer 135(10):2404–2412. doi:10.1002/ijc.28876
Schache AG, Liloglou T, Risk JM et al (2011) Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin Cancer Res 17:6262–6271
Schlecht NF, Brandwein-Gensler M, Nuovo GJ et al (2011) A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol 24:1295–1305
Shi W, Kato H, Perez-Ordonez B et al (2009) Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol 27:6213–6221
Singhi AD, Westra WH (2010) Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 116:2166–2173
Smeets SJ, Hesselink AT, Speel EJ et al (2007) A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 121:2465–2472
Smith EM, Wang D, Kim Y, Rubenstein LM, Lee JH, Haugen TH, Turek LP (2008) P16INK4a expression, human papillomavirus, and survival in head and neck cancer. Oral Oncol 44(2):133–142. doi:10.1016/j.oraloncology.2007.01.010
Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ (2013) Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol 2(1):51–61. doi:10.5539/cco.v2n1p51
Talis AL, Huibregtse JM, Howley PM (1998) The role of E6AP in the regulation of p53 protein levels in human papillomavirus (HPV)-positive and HPV-negative cells. J Biol Chem 273:6439–6445
Termine N, Panzarella V, Falaschini S et al (2008) HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: a meta-analysis (1988–2007). Ann Oncol 19:1681–1690
Tinhofer I, Johrens K, Keilholz U et al (2015) Contribution of human papilloma virus to the incidence of squamous cell carcinoma of the head and neck in a European population with high smoking prevalence. Eur J Cancer
Tran N, Rose BR, O’brien CJ (2007) Role of human papillomavirus in the etiology of head and neck cancer. Head Neck 29:64–70
Upile NS, Shaw RJ, Jones TM et al (2014) Squamous cell carcinoma of the head and neck outside the oropharynx is rarely human papillomavirus related. Laryngoscope 124:2739–2744
Weinberger PM, Merkley MA, Khichi SS, Lee JR, Psyrri A, Jackson LL, Dynan WS (2010) Human papillomavirus-active head and neck cancer and ethnic health disparities. Laryngoscope 120(8):1531–1537. doi:10.1002/lary.20984
Weinberger PM, Yu Z, Haffty BG et al (2006) Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 24:736–747
Weiss D, Koopmann M, Rudack C (2011) Prevalence and impact on clinicopathological characteristics of human papillomavirus-16 DNA in cervical lymph node metastases of head and neck squamous cell carcinoma. Head Neck 33:856–862
Westra WH (2014) Detection of human papillomavirus (HPV) in clinical samples: evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol 50:771–779
Wiest T, Schwarz E, Enders C et al (2002) Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 21:1510–1517
Wittekindt C, Gultekin E, Weissenborn SJ, Dienes HP, Pfister HJ, Klussmann JP (2005) Expression of p16 protein is associated with human papillomavirus status in tonsillar carcinomas and has implications on survival. Adv Otorhinolaryngol 62:72–80. doi:10.1159/000082474
Xie X, Piao L, Bullock BN et al (2013) Targeting HPV16 E6-p300 interaction reactivates p53 and inhibits the tumorigenicity of HPV-positive head and neck squamous cell carcinoma. Oncogene
Xu Y, Liu S, Yi H, Wang J, Luo Y, Yin S (2015) Low prevalence of human papillomavirus in head and neck squamous cell carcinoma in Chinese patients. J Med Virol 87(2):281–286. doi:10.1002/jmv.24052
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coordes, A., Lenz, K., Qian, X. et al. Meta-analysis of survival in patients with HNSCC discriminates risk depending on combined HPV and p16 status. Eur Arch Otorhinolaryngol 273, 2157–2169 (2016). https://doi.org/10.1007/s00405-015-3728-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-015-3728-0