Abstract
We aimed to evaluate the impact of concurrent chemoradiotherapy (CCRT) on the survival of patients with squamous cell carcinoma of the temporal bone. We retrospectively analyzed the data of 13 consecutive patients who were treated by definitive radiation therapy (RT) or CCRT as the initial treatment between 1999 and 2012. There were 5 patients with stage II disease, 5 with stage III, and 3 with stage IV, as classified according to the University of Pittsburgh system. Among these, 2, 4, and 3 patients, respectively, were treated by CCRT; whereas the remaining (3 patients with stage II and 1 with stage III) were treated by RT alone. Median follow-up duration was 39 months (12–106 months) in all cases, and 61.5 months (17–70 months) in censored cases. The 5-year overall survival (OS) rates were 51 % in all patients, and 40, 100, and 0 % in patients with stage II, stage III, and stage IV disease, respectively. In patients with stage II and III disease, the 5-year OS rates were 80 % in the CCRT group and 50 % in the RT-alone group. We found better prognosis in patients with stage II and III disease who were treated by CCRT. Only 2 patients treated by CCRT experienced adverse events more than grade 3, which were neutropenia and dermatitis. There was no late adverse event of bony necrosis. Our study results indicate that CCRT is safe and very effective as a first-line treatment for stage II and III squamous cell carcinoma of the temporal bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Squamous cell carcinoma rarely occurs in the external auditory canal and middle ear, with a reported prevalence of 1 per million [1, 2]. Thus, it has been difficult to analyze data to formulate an optimal evaluation and treatment strategy. Currently, there is no staging system for external auditory canal accepted by the American Joint Committee on Cancer or International Union Against Cancer. This lack of a universally accepted staging system poses a critical problem when attempting to compare treatment strategies and outcomes among multiple institutions [1, 3, 4].
The reliability of radiologic evaluation of disease extent, surgical procedures, and efficacy of radiation therapy (RT) are still controversial[5–7]. Anatomic complexity makes surgical resection relatively challenging, especially for advanced cases that have invaded the temporal bone and the surrounding tissues [8–10]. As the surgical margins of these tumors are hard to estimate and are not obvious in some cases, pathological examination occasionally reveals tumor-positive surgical margins, necessitating post-operative RT in most cases [11, 12]. Moreover, radical surgery, which involves resection of the temporal bone to some extent, often results in an unfavorable quality of life due to facial nerve palsy, hearing impairment, and so on.
Concurrent chemoradiotherapy (CCRT) has already been recognized as a very effective treatment for head and neck squamous cell carcinoma [13]. Since 1999, our center has treated patients with squamous cell carcinoma of the temporal bone mainly by CCRT as the first-line treatment, in order to preserve organs and functions. In this retrospective single-center experience, we aimed to assess the effectiveness of definitive RT, with or without chemotherapy, as the first-line treatment for squamous cell carcinoma of the temporal bone.
Materials and methods
Patients
Thirteen consecutive, previously untreated, patients with squamous cell carcinoma of the temporal bone underwent RT at Kanagawa Cancer Center between 1999 and 2012.
Patients were diagnosed and staged according to the University of Pittsburgh system (1): T1, tumor limited to the external auditory canal without bony erosion or evidence of soft tissue extension; T2, tumor with limited external auditory canal bony erosion (not full thickness) or radiographic finding consistent with limited (<0.5 cm) soft tissue involvement; T3, tumor eroding the osseous external auditory canal (full thickness) with limited (<0.5 cm) soft tissue involvement or tumor involving middle ear and/or mastoid, or patients presenting with facial paralysis; T4, tumor eroding the cochlea, petrous apex, medial wall of middle ear, carotid canal, jugular foramen or dura, or with extensive (>0.5 cm) soft tissue involvement. Regional lymph node involvement results in upstaging to stage III (T1, N1) or stage IV (T2, T3, T4, N1).
Assignment of stage was based on clinical, computed tomography (CT), magnetic resonance imaging (MRI), and 18-fluoro-2-deoxyglucose positron emission tomography (FDG-PET) findings.
Treatment
As an initial treatment, all patients were treated by RT, with or without chemotherapy. Each patient received 2 Gy/fraction RT every weekday (5 fractions per week) with a total dose of 70 Gy. Two dimensional radiation therapy (2D RT) or three-dimensional conformal radiation therapy (3D CRT) were used.
Concurrent chemoradiotherapy was given to patients under 75 years of age in order to improve locoregional control and survival. The chemotherapeutic regimen was 2 cycles of PF, which consisted of continuous intravenous administration of 1000 mg/m2 of 5-fluorouracil (5-FU) from day 1 through day 4, and a bolus injection of 60 mg/m2 of cisplatin (CDDP) on day 4. Patients who could not receive PF due to renal dysfunction were given 7 cycles of weekly docetaxel (15 mg/m2/week). For unresectable cases, induction chemotherapy with 3 cycles of TPF (docetaxel 60 mg/m2 on day 1, CDDP 70 mg/m2 on day 4, 5-FU 1000 mg/m2 on days 1–5) was given before CCRT.
Statistical analysis
Survival rates were estimated by the Kaplan–Meier product limit method. Comparisons between subgroups were performed by the log-rank test.
Results
Patient characteristics are summarized in Table 1. Median age was 64 years (range 41–87 years), and male-to-female ratio was 8:5. There were 5 patients with stage II disease, 5 with stage III, and 3 with stage IV. Two patients in stage IV were unresectable cases.
Treatment regimen and outcomes of 13 patients are summarized in Table 2. Of the total study population, 2/5 patients with stage II, 4/5 patients with stage III, and all (3/3) patients with stage IV were treated by CCRT, whereas the rest by RT alone.
The chemotherapeutic regimen for 4 patients with stage II disease, 2 with stage III, and 1 with stage IV was 2 cycles of PF (cisplatin and 5-FU). Of the remaining 2 patients with stage IV disease, 1 was treated by 7 cycles of weekly docetaxel and another by induction chemotherapy with 3 cycles of TPF followed by only 1 cycle of PF due to grade 2 renal dysfunction. A median dose of 68 Gy (range 62–70 Gy) was given. Median follow-up duration was 39 months (range 12–106 months) in all cases and 61.5 months (range 17–70 months) in censored cases.
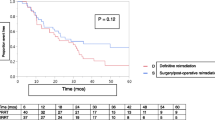
The 5-year overall survival (OS) rate in all patients was 51 % (Fig. 1). The 5-year OS rates were 40, 100, and 0 % for patients with stage II, III and IV disease, respectively. Compared with the combined OS rates of stage II and stage III, stage IV tumors had significantly worse prognosis (67 vs. 0 %, respectively; p < 0.001) (Fig. 2). In stage II and III patients, the 5-year OS rates were 80 % in the CCRT group and 50 % with RT alone (Fig. 3).
Figure 4 shows a patient example with contrast-enhanced CT-scan before therapy, the 3D-conformal RT-treatment plan and the results in CT post CRT. In this patient the concurrent chemoradiation with 5-FU/Cisplatin plus 62 Gy was highly effective. Acute toxicities more than grade 3 were seen only in two patients, one showed neutropenia grade 4 and one developed severe dermatitis. Downgraded hearing was noticed in all patients after treatment but unfortunately there are no audiometric data during the follow-up period available. None of the patients developed facial nerve paralysis or bone necrosis.
Case 6. a Computed tomography (CT) findings before treatment; the tumor had invaded middle ear and mastoid and parotid gland. b Treatment plan. c CT 2 months after treatment; concurrent chemoradiotherapy (CCRT) with two course of PF (cisplatin and 5-fluorouracil regimen) and 62 Gy of radiation therapy had been completed and the tumor had almost disappeared
Discussion
Squamous cell carcinoma of the temporal bone is a rare but aggressive tumor, which usually has a poor prognosis and a 5-year OS rate ranging from 38 to 67 % [5, 6, 11, 12, 14, 15]. In addition, published results are difficult to interpret because there is no universally accepted staging system. Although Clark’s modification of Stell’s staging proposal has been used in the past, the Pittsburgh classification system is the most commonly used at present [1, 3, 4].
The reliability of radiologic evaluation of disease extent, surgical procedures, and efficacy of RT are still matters of controversy. Most authors have advocated some form of surgical resection, with adjuvant RT for advanced stage disease.
The extent and type of surgery for such tumors have been greatly debated in literature, ranging from simple resection to complete en bloc removal of the temporal bone, depending on the extent of the tumor [8–10, 14, 16]. Different procedures have been described for the management of T1 and T2 tumors—from local resection to subtotal petrosectomy [17, 18].
According to the University of Pittsburg system, the 5-year OS rates for patients who were treated by surgery, with or without adjuvant chemotherapy, were 65–100 % for stage I, 65–100 % for stage II, 30–70 % for stage III, and 0–50 % for stage IV [5, 12, 19–21].
In advanced cases, however, radical surgery often results in an unfavorable quality of life due to facial nerve paralysis, hearing impairment, cosmetic disorder, and so on [8, 22]. Prasad et al. [23] also reported that patients with advanced tumors are at a very high surgical risk, with a perioperative mortality risk of 18.7 % for total temporal bone resection or subtotal temporal bone resection.
At our center, we have treated patients with squamous cell carcinoma of the temporal bone mainly by CCRT as first-line treatment, to preserve organs and functions. In our series, the 5-year OS rates for patients with stage II, stage III, and stage IV disease were 40, 100, and 0 %, respectively. The lower survival rate of stage II compared with stage III could be explained by the fact 3 patients in stage II were treated by RT alone, and 2 of them had recurrence.
In patients with stage II and III disease, the 5-year OS rates were 80 % in the CCRT group and 50 % in the RT-alone group. Patients with stage II and III disease treated by CCRT had good prognosis. There was no statistical difference between CCRT and RT alone in our very small group. In 6 patients with stage II and III disease who received CCRT, recurrence occurred in only 1 patient and this was from involvement of a lymph node that was out of the radiation field. Therefore, we consider that CCRT was very effective for patients with stage II and III disease.
In patients with stage IV disease, we experienced very poor prognosis (the 5-year OS rate was 0 %) but this result should be interpreted with caution because two of three patients were unresectable cases and they received differently regimen (one with low dose DOC vs. one with PF vs. one with TPF induction chemotherapy + PF) in our series. We might need to reconsider treatment approach for stage IV disease in terms of chemotherapeutic regimen or radiation methods because there was the report that showing good prognosis in patients with stage IV disease who were treated by other regimen [7].
We found that 5/13 (38 %) patients had local recurrence, with an overall 5-year local control (LC) rate of 62 %. Specifically, 2 patients with stage II disease who received RT alone and 3 patients with stage IV disease had local recurrence. There was no local failure in patients with stage II and III who received CCRT. Nodal recurrence out of the radiation field occurred in 1 patient with stage II who received CCRT (Table 2).
One patient with stage IV disease who underwent salvage surgery at the primary site for tumor recurrence died of distant metastasis. The other 2 with stage IV disease were given palliative care because of unresectable tumor. Two patients with stage II disease refused salvage surgery and instead had stereotactic RT for local or nodal recurrence; however, they died of uncontrolled disease. The remaining patient with stage II, who also refused salvage surgery, received palliative chemotherapy.
Some investigators reported that recurrence resulted to a worse survival [5, 11]. In our series, we obtained the same result: the 5-year OS rate was significantly worse in patients with recurrence compared with those without recurrence (0 vs. 100 %, respectively; p < 0.001). This highlights the significance of selection of an appropriate first-line treatment for this tumor.
Concurrent chemoradiotherapy for head and neck squamous cell carcinoma has already been recognized as a very effective treatment [13]. There were some reports on definitive RT as the first-line treatment for temporal bone carcinoma; in addition, there had been no reports showing that RT was inferior to surgical treatment for both early and advanced cases [5, 7, 14, 15]. It is thought that CCRT is a safe treatment as there have been no reports on treatment-related deaths.
In patients who underwent surgery, pre- or post-operative RT is often conducted. Min et al. [5] reported that 70 % patients had received perioperative RT; even in patients with early-stage disease, more than 50 % had received perioperative RT. Fang-Lu et al. [12] also reported that 92 % of patients who underwent surgical procedures received adjuvant RT.
Adjuvant RT was given to most cases, including early-stage cases, which underwent surgical operation. Thus, from the viewpoint of organ and function preservation, if treatment outcome was not significantly different between the surgery-based treatment group and radiation-based treatment group, CCRT can become one of the first-line treatment choices.
Recently, Takenaka et al. [24] performed a meta-analysis on the effects of CCRT on temporal bone cancer. They compared patients with stage III and stage IV disease who received either surgery ± RT, preoperative CCRT, definitive CCRT, or post-operative CCRT, and found 5-year OS rates to be 53.5, 85.7, 43.6, and 0 %, respectively. Their findings suggested that CCRT might improve the survival of surgically treated patients with temporal bone cancer, and that the 5-year OS rate of the definitive CCRT group was comparable with that of the standard treatment (surgery ± RT) group. Of note, some of the definitive CCRT cases were surgically unresectable cases, but unfortunately in their analysis, there was no data based on the results of only resectable cases.
In our series, the 5-year OS rate of patients with resectable stage III was 100 %, indicating a good prognosis; thus, CCRT may be an effective treatment for resectable cases. Further studies must evaluate the efficacy of definitive CCRT as the first-line treatment for advanced resectable temporal bone cancer.
In our series, in addition, 2D RT and 3D CRT were used for treatment. However, recently 2D RT is no acceptable clinical standard for radiotherapy of temporal bone squamous cell carcinoma. We should use highly conformal RT techniques like IMRT (intensity modulated radiation therapy), IGRT (image guided radiation therapy), or SRT (stereotactic radiation therapy) to obtain improvements in homogeneity and dose distribution with possible minimizing the dose to critical structures.
Conclusion
Concurrent chemoradiotherapy with the use of a PF regimen is a safe and effective first-line treatment for patients with squamous cell carcinoma of the temporal bone, especially for stage II and III tumors. It is important to evaluate the effect of definitive CCRT for resectable advanced cases.
References
Arriaga M et al (1990) Staging proposal for external auditory meatus carcinoma based on preoperative clinical examination and computed tomography findings. Ann Otol Rhinol Laryngol 99(9 Pt 1):714–721
Arena S, Keen M (1988) Carcinoma of the middle ear and temporal bone. Am J Otol 9(5):351–356
Hirsch BE (2002) Staging system revision. Arch Otolaryngol Head Neck Surg 128(1):93–94
Clark LJ et al (1991) Squamous carcinoma of the temporal bone: a revised staging. J Laryngol Otol 105(5):346–348
Yin M et al (2006) Analysis of 95 cases of squamous cell carcinoma of the external and middle ear. Auris Nasus Larynx 33(3):251–257
Ogawa K et al (2007) Treatment and prognosis of squamous cell carcinoma of the external auditory canal and middle ear: a multi-institutional retrospective review of 87 patients. Int J Radiat Oncol Biol Phys 68(5):1326–1334
Shiga K et al (2011) Concomitant chemoradiotherapy as a standard treatment for squamous cell carcinoma of the temporal bone. Skull Base 21(3):153–158
Lewis JS (1983) Surgical management of tumors of the middle ear and mastoid. J Laryngol Otol 97(4):299–311
Kinney SE, Wood BG (1987) Malignancies of the external ear canal and temporal bone: surgical techniques and results. Laryngoscope 97(2):158–164
Pensak ML et al (1996) Temporal bone carcinoma: contemporary perspectives in the skull base surgical era. Laryngoscope 106(10):1234–1237
Gidley PW, Roberts DB, Sturgis EM (2010) Squamous cell carcinoma of the temporal bone. Laryngoscope 120(6):1144–1151
Chi FL et al (2011) Survival outcomes in surgical treatment of 72 cases of squamous cell carcinoma of the temporal bone. Otol Neurotol 32(4):665–669
Pignon JP et al (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 355(9208):949–955
Zhang B et al (1999) Squamous cell carcinoma of temporal bone: reported on 33 patients. Head Neck 21(5):461–466
Pemberton LS, Swindell R, Sykes AJ (2006) Primary radical radiotherapy for squamous cell carcinoma of the middle ear and external auditory cana–an historical series. Clin Oncol (R Coll Radiol) 18(5):390–394
Lewis JS (1975) Temporal bone resection. Review of 100 cases. Arch Otolaryngol 101(1):23–25
Golding-Wood DG, Quiney RE, Cheesman AD (1989) Carcinoma of the ear: retrospective analysis of 61 patients. J Laryngol Otol 103(7):653–656
Goodwin WJ, Jesse RH (1980) Malignant neoplasms of the external auditory canal and temporal bone. Arch Otolaryngol 106(11):675–679
Chang CH et al (2009) Treatments and outcomes of malignant tumors of external auditory canal. Am J Otolaryngol 30(1):44–48
Moody SA, Hirsch BE, Myers EN (2000) Squamous cell carcinoma of the external auditory canal: an evaluation of a staging system. Am J Otol 21(4):582–588
Austin JR, Stewart KL, Fawzi N (1994) Squamous cell carcinoma of the external auditory canal. Therapeutic prognosis based on a proposed staging system. Arch Otolaryngol Head Neck Surg 120(11):1228–1232
Manolidis S et al (1998) Temporal bone and lateral skull base malignancy: experience and results with 81 patients. Am J Otol 19(6 Suppl):S1–S15
Prasad S, Janecka IP (1994) Efficacy of surgical treatments for squamous cell carcinoma of the temporal bone: a literature review. Otolaryngol Head Neck Surg 110(3):270–280
Takenaka Y et al (2014) Chemoradiation therapy for squamous cell carcinoma of the external auditory canal: a meta-analysis. Head Neck. doi:10.1002/hed.23698
Conflict of interest
All author report no conflict of interest related to his manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitani, Y., Kubota, A., Furukawa, M. et al. Primary definitive radiotherapy with or without chemotherapy for squamous cell carcinoma of the temporal bone. Eur Arch Otorhinolaryngol 273, 1293–1298 (2016). https://doi.org/10.1007/s00405-015-3616-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-015-3616-7