Abstract
The objective of this study was to evaluate differences in gustatory function in smokers of both sexes and identify any differences in the shape, density and vascularisation of the fungiform papillae (fPap) of smokers’ tongue. Additional aim was to investigate any relation between the age, pack years and differences in shape, density, vascularization of fPap and sex. In 166 smokers (81 males, 85 females, age range 20–80 years), divided in age groups, electrogustometry (EGM) thresholds at the chorda tympani area, at the soft palate area and at the area of the vallate papillae were recorded bilaterally. Morphology and density of the fPap and blood vessels’ density and morphology at the tip of the tongue were examined using contact endoscopy (CE). EGM thresholds of all smoking subjects tended to increase compared to the non-smoking participants. Morphology, vascularization and density of fPap were found to be worse in smokers than in non-smokers. Interestingly, some participants, despite having increased number of pack years, tended to have almost similar EGM thresholds with non-smoking subjects of the same age group. Smoking tends to affect density, morphology and vascularization of the fPap. There is a correlation between the duration of smoking (pack years) and the afore-mentioned parameters. The use of τ-Kendall criterion provided useful information about the different correlation between the EGM thresholds and vascularization, the EGM thresholds and morphology of fPap and EGM thresholds and density of fPap. The majority of smokers had elevated EGM thresholds compared to non-smokers. Smoking is an important factor which can lead to decreased taste acuity. The combination of methods, such as EGM and CE, can provide useful information about the morphology and function of taste buds. Of interest, women are less affected than men, irrespective of the age group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungiform papillae (fPap) are located on the dorsal surface of the tongue in humans and are more numerous at its tip than at any other tongue area [1]. Gustatory stimuli’s thresholds are related to the precise location of stimulation and to the number of fPap at the stimulated tongue area [2]. Changes in shape and vascularization of fPap under various circumstances have been also studied in the past [3]. The effects of smoking on taste [4] and olfaction have been studied extensively. However, few studies have provided morphological data about the effects of cigarette smoking on the size and shape of the tongue papillae. The aim of the present study was to investigate if there is a difference between smokers and non-smokers in electrogustometry (EGM) thresholds recorded at the anterior and posterior tongue and soft palate. An additional aim was to test, if any eventually observed difference in EGM thresholds at the anterior tongue between smokers and non-smokers may be associated with any difference in density and/or morphology of fPap at that anatomical site.
Patients and methods
One-hundred and sixty-six smokers (81 males and 85 females) participated in the study. The subjects were divided in age groups, as proposed previously [5] (Table 1 ). Criteria for exclusion from the study were a positive history for disorders affecting gustatory function, a history of diabetes mellitus, use of antihypertensive drugs (such as captopril and ramipril), prior otological operations and neurological disorders (including facial palsy). The EGM thresholds of the smokers were compared to those of non-smoking participants (n = 166, males = 81, females = 85) with the same age distribution. Volunteers participated in the study after they had been informed of its background and purpose and after they had provided written consent. The study protocol was reviewed and approved by the Institutional Review Board of The Aristotle University of Thessaloniki, Greece and was in accordance with the principles of the Helsinki Declaration. To minimize variability resulting from the examination technique, all examinations were carried out by the same examiner (PP). Four male smokers of the age group 20–29 years, two male smokers of the age group 30–39 and two of the age group 50–59 years reported experiencing bad taste sensation without any respective gustatory stimulus (phantogeusia) or reduced taste sensation (hypogeusia). Two female smokers of the age group 30–39 years reported hypogeusia and another two of the age group 50–59 years reported phantogeusia. All smokers reported that they kept their cigarette usually in the centre of their lips. Table 1 provides information about the duration of smoking and the number of smoked cigarettes per day. One-hundred and sixty-six non-smoking individuals (81 males and 85 females) of different ages whose data were previously published served as controls [6]. We have used the same age groups to provide reliable age-matched data [6].
Electrogustometry testing
Taste acuity was evaluated with EGM. Electrical stimuli were delivered with an electrogustometer (TR-06, Rion Co, Tokyo, Japan) with a single, flat, circular stainless steel stimulus probe (5 mm in diameter). The device produces low-amplitude stimuli of pre-determined duration (0.5, 1, 1.5 and 2 s). A feedback circuit controls the output current with an error quote of <1 % [7].
All subjects were instructed not to smoke, drink or eat for 1 h before beginning the testing session. First, a 30 dB-stimulus was administered to test whether the subject was in a position to recognize electrogustometric stimuli. Stimulation started at the lowest stimulus amplitude (−6 dB) and increasingly stronger stimuli were presented until the subject recognized the stimulus. If the threshold for stimulus perception was not clearly determined, the next higher- and lower-strength stimuli were presented to the individual. The electric threshold scores were measured at six locations, namely para-medially on both sides of the tongue apex (each 2 cm away from the tip) at an area innervated by the chorda tympani, at the area of the vallate papillae on both sides of the tongue (innervated by the glossopharyngeal nerve) and at the soft palate (area innervated by the major petrosal nerve) bilaterally, as reported previously [6]. A 500-ms electric stimulus was applied, beginning at −6 dB and increasing up to +34 dB (3–400 μΑ) in 2 dB steps. Thresholds were measured randomly on both sides of the tongue to avoid any possible bias. All six areas were tested applying the same stimulus duration before proceeding using different stimulus duration. This procedure resulted in a 3–4 min stimulus interval, thereby decreasing the possibility of emergence of stimulus adaptation. The subjects had been instructed to discriminate between the perception of a sour/metallic taste (suggesting gustatory function––taste threshold) and the perception of an electrical sensation (suggesting trigeminal stimulation). The subjects answered “yes” or “no”, if they perceived any taste sensation. Subjects were kept unaware of whether or not any electrical stimulus was applied (blinded test) as done in other studies [6]. A two-alternative forced-choice initially ascending single-staircase detection protocol was applied using a two-down, one-up rule [8]. The trial sequence was begun at the 8-mA current level, as in previous studies [8]. If subjects missed a trial before reaching this criterion, the subsequent trial was presented at the next higher stimulus level. The latter process was continued until five consecutive correct trials occurred at a given current level. At this point, the subsequent trial was presented at the next lower stimulus level. If the first or the second two successive correct trials were missed at this stimulus level, the subsequent trial was presented at the next higher level, representing a reversal in the staircase. If two successive correct trials occurred at this level, the following trial was given at the next lower stimulus level [9].
Contact endoscopy
Contact endoscopic (CE) imaging was performed using a 30° contact endoscope (magnification 60 × and 150 × ; Karl Storz, Tuttlingen, Germany). Identification of fPap was first performed using a non-contact technique. Subjects were instructed to rinse their mouth with water before contact endoscopy. A CE technique was first used without staining for imaging of subepithelial vessels. After careful suctioning of the saliva, methylene-blue 1 % solution was used to stain epithelia and taste pores. A filter paper strip delineating an area of 1 cm2 was placed in a para-median position on the tongue tip as proposed by Negoro et al. [5]. To address the problem of continuous movement of the tongue during CE and to avoid venous congestion and hyperemia which could eventually confound CE findings, volunteers were asked to keep the tongue in a fixed position as much as possible and were advised to bite gently the tip of their tongue between their upper and lower teeth. Moreover, subjects were asked to seat in the examination chair with their head and neck supported by a pillow. Examination time by means of CE was about 30 s. No local anesthesia was necessary. A cold light source was used to minimize any heat produced at the tip of the endoscope. No changes (increase or decrease) in vascularization due to application of the endoscope on the mucosa have been observed during examination by CE.
The form of the fPap was classified to one of the four types according to the following classification paradigm introduced by Negoro et al. [5]: Type 1 (egg-shaped or long ellipse type––without surface thickness), Type 2 (slight thicker surface compared to Type 1), Type 3 (thick and irregular surface) and Type 4 (remarkably flat and atrophic surface). Type 1 corresponds to the healthier state and Type 4 to the most pathological state. It should be noted that mushroom-shaped papillae with horny tips were counted as filiform (and not as fungiform) papillae. Due to their very light staining, fPap could be readily distinguished from filiform papillae, which stained dark [3]. For evaluation of the density of fPap (number of papillae per cm2), the CE image with the highest fPap density taken from each individual was used. The classification of the blood vessels’ morphology at the tongue apex was also performed according to Negoro et al. [5]: Type A (clear loop and wooden branch shape), Type B (unclear loop and wooden branch shape), Type C (elongated blood vessels), Type D (granular shape or dotted shape) and Type E (unclear blood vessels). Type A represents the “healthiest” morphology and Type E corresponds to the “most pathological” morphology. All volunteers completed the study. The findings, and particularly the CE images and the classification of the fPap, were double-checked by two investigators (P.P. and G.K.) to achieve consensus and minimize any possible mistakes.
Statistical analysis
The null hypothesis was that there was no statistical difference in EGM thresholds between age groups and between sexes. For statistical analysis’ computational purposes, if an EGM threshold could not be measured at all, then it was assigned a numerical value of 36 dB. To examine if the numerical values of the measurement results were normally distributed, a quantile–quantile plot (QQ plot) was applied. This QQ plot analysis resulted in the distribution not being normal. Therefore, non-parametric tests were applied. The level of statistical significance was set at p < 0.05. On each occasion, EGM thresholds between the two groups were compared using Kruskal–Wallis and Mann–Whitney tests. The Bonferroni correction was used as necessary. Tukey’s multiple comparison test was used to detect differences significant at the 0.05 level in mean thresholds for the various age categories. For analysis of the regression between age, form and vascularisation of fPap, the Kendall rank correlation coefficient was applied. The null hypothesis was that the two variables examined on each occasion were independent. The results were analyzed using SPSS software (version 12 for Windows, SPSS Inc. Chicago, IL, USA). No statistical analysis in the age group >70 years was done because of the small number of participants in this particular group.
Results
EGM thresholds
Table 2 depicts the EGM thresholds’ differences of smokers and non-smokers of both sexes in all age groups. Of interest, EGM thresholds show a definite tendency to increase with increasing age in smokers of both sexes. Females tend to have lower EGM thresholds than males, although in age groups 40–49 and 50–59 years some subjects present quite similar EGM thresholds. There are significant statistical differences (p < 0.05) between smokers and non-smokers, both males and females. We have also observed statistical differences in the EGM thresholds between the two sexes. Differences have been observed at various sites on the tongue as depicted in Table 2. Male smokers tend to have higher EGM thresholds than female smokers in almost all tested loci.
Fungiform papillae structure
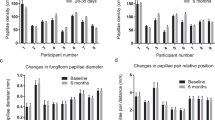
Changes in shape and density of fPap as well as in vascularization of the tip of the tongue were evaluated by means of CE. No change in vascularization or shape has been observed during the examination. Shape of fPap and vascularization of the tongue tip worsened significantly in smokers, as it can be observed with the use of Negoro’s classification, in individuals of all age groups. Changes in density of fPap were present in both smoking men and women in a rather non-uniform manner as a function of age, as depicted in Fig. 1. Smokers showed a lower fPap density than non-smokers of both sexes. Table 3 depicts the proportions (as a percent of total, %) of fPap classes according to the shape and vascularization in non-smoking and smoking participants of both sexes of all age groups.
Correlation between EGM thresholds and fungiform papillae structure
Calculation of the Kendall rank correlation coefficient disclosed a strong relationship between EGM thresholds and vascularisation of the tip of the tongue in both females and males, EGM thresholds and vascular shape at the tip of the tongue and taste thresholds and density of the fPap. A definite stronger correlation with increasing age has been observed. As shown in Table 4 there is a stronger correlation between EGM thresholds and vascularization, EGM thresholds (at Rapex and Lapex) and shape of fPap and EGM thresholds and fPap density in males than in females of both smokers’ and non-smokers’ groups. The calculation of the Kendall rank correlation coefficient shows that the correlation between EGM thresholds and shape and EGM thresholds and fPap density is stronger in smokers of both sexes.
Discussion
The present study made use of techniques that may be easily implemented in both an inpatient and outpatient clinical setting. EGM can be used in the clinical routine for a variety of clinical purposes, including the evaluation of taste acuity in patients suffering from various associated diseases.
Nicotine has been associated in the past with alteration of gustatory function under conditions of chronic exposure. The present study included participants of both sexes, divided in different age groups of both smokers and a respective control group of non-smokers, to increase the level of evidence of the results. It has been found that EGM thresholds increase with advancing age, starting at the age of 60 years, in the areas innervated by the chorda tympani and glossopharyngeal nerves and starting at the age of 70 years, in the area innervated by the greater petrosal nerve [4].
Statistically significant differences were detected between the EGM thresholds of smokers and non-smokers. In other studies, EGM thresholds were significantly higher in smokers, especially older ones, than in non-smokers. The findings of the present study suggest that changes in taste thresholds begin relatively early in life and they are independent of the age of the smoker. In a previous study [10], 38 smokers (mean age 37 years) and 34 non-smokers (mean age 33.5 years) were examined with contact endoscopy and chemogustometry (taste strips). The parameters assessed were the number of fPap per square centimeter in a noncontact way and their morphology (surface, capillary vessels) by contact endoscopy. The morphology of the filiform papillae has also been assessed. In addition, clinical testing of gustatory function was performed by means of taste strips and subjective intensity ratings of natural taste stimuli. The authors found no significant difference in clinical testing and intensity ratings between the two study groups. A trend toward significance was found in taste strip results for decreased bitter taste in heavy smokers (p = 0.06). The number and the size of fPap did not significantly differ between the study groups. No sex-related differences were observed. These authors have used taste strips for functional tasting. Chemogustometry (CGM) has a qualitative character that does not have the same test–retest reliability as EGM. In a previous study [11], chemogustometry was used to evaluate taste disorders in smokers. For that purpose, 1,312 participants were examined. Approximately, 20 % recognized only three or less of the four tastes when presented at supra-threshold concentrations, indicating signs of taste impairment. Heavy smokers of 20 or more cigarettes/day had significantly increased risk for taste impairment. The respective differences in “Results” may result from the different methodologies (EGM and CGM).
The use of CE provided the advantage to study the vascularization of the tongue tip and the shape of the fPap. Results of this study suggest that these two parameters are significantly associated with taste acuity as assessed by EGM. Nonetheless, it should be stressed again that not all fPap contain taste buds and, therefore, not all fPap produce taste sensation [1, 2] and that there are variations in sensitivity of fPap to chemical stimuli [3]. Our endoscopic findings also disagree with those of Konstantinidis et al. [10]. In their study, the number and the size of fPap did not significantly differ between the study groups. In a previous study [12], 62 male subjects (34 non-smokers and 28 smokers) who served in the military forces were randomly chosen. Taste thresholds were also measured with EGM. The morphology and density of the fPap at the tip of the tongue were examined with contact endoscopy (CE). Differences concerning the shape and the vessels of the fPap between the groups were also detected. Fewer and flatter fPap were found in 22 smokers (79 %).
A finding which needs further investigation is the observed differences between the two sides of the tongue. In the present study, lower EGM thresholds were recorded on the right side of the tongue, with only few exceptions in various age groups in both smokers and non-smokers. In previous study [6], similar results were found, namely statistically significant differences in EGM thresholds between the right and the left side of the tongue and between the two sexes. Aging was associated with a progressive increase in EGM thresholds.
Additionally, some smokers of various age groups had normal EGM thresholds despite the fact that during (at least) the last 2 years before testing they had been smoking a considerable number of cigarettes (~20 per day). Of note, it has been reported that male smokers in their fourth and fifth decades of life show significantly lower taste thresholds at their soft palate compared to non-smokers [13]. It may be that both findings are due to changes in the shape and vascularization which may not be of the same degree in all smokers or that the cell turnover in some smokers is similar to that of the non-smokers. The latter hypothesis has been proposed in a previous study, in which the long-term effects of nicotine were studied on rat fungiform taste buds [13].
Despite the significant differences in shape and vascularisation of the fPap, there were no differences found concerning the density of papillae between the two groups. It has been suggested that long-term exposure of taste buds to nicotine leads to significant reduction in their size [18]. However, changes in shape and size of the papillae are not accompanied by any simultaneous significant change in their number [14]. Therefore, it is important to identify specific factors that account for the variability in initial nicotine sensitivity in humans. Such factors could increase our understanding of the etiology of dependence, as well as supporting the efforts of prevention of tobacco use, especially in teenagers. One important individual difference may be due to gender. On measures of reward and reinforcement, women may be less responsive than men to manipulations of nicotine exposure, while women may be more responsive than men to manipulations of non-nicotine components of cigarette smoking, such as cues [15, 16]. However, because virtually all of this research has been conducted with dependent addicted smokers, it is not clear whether the sex difference in sensitivity to nicotine results from chronic exposure over years of regular smoking or may be present from the start of experimentation with smoking in teens.
This study supports the use of the combination of EGM and CE methods for the study of taste disorders in smokers. Both techniques provide useful clinical data in a short time frame and exhibit good test–retest reliability.
References
Miller IJ, Reedy FE (1990) Variations in human taste bud density and taste intensity perception. Physiol Behav 47:1213–1219
Miller SL, Mirza N, Doty R (2002) Electrogustometric thresholds: relationship to anterior tongue locus, area of stimulation and number of fungiform papillae. Physiol Behav 75:753–757
Just T, Pau WH, Witt M, Hummel T (2006) Contact endoscopic comparison of morphology of human fungiform papillae of healthy subjects and patients with transected chorda tympani nerve. Laryngoscope 116:1216–1222
Nin T, Sakagami M, Sone-Okunaka M, Muto T, Mishiro Y, Fukazawa K (2006) Taste function after section of chorda tympani nerve in middle ear surgery. Auris Nasus Larynx 33:13–17
Negoro A, Umemoto M, Fukazawa K, Terada T, Sakagami M (2004) Observation of tongue papillae by video microscopy and contact endoscopy to investigate their correlation with taste function. Auris Nasus Larynx 31:255–259
Pavlidis P, Gouveris H, Anogeianaki A, Koutsonikolas D, Anogianakis G, Kekes G (2013) Age-related changes in electrogustometry thresholds, tongue tip vascularization, density, and form of the fungiform papillae in humans. Chem Senses 38:35–43
Tomita H, Ikeda M, Okuda Y (1986) Basis and practice of clinical taste examinations. Auris Nasus Larynx 13(Suppl 1):1–15
Loucks CA, Doty RL (2004) Effects of stimulation duration on electrogustometric thresholds. Physiol Behav 81:1–4
Lemon CH, Smith DV (2005) Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol 94:3719–3729
Konstantinidis I, Chatziavramidis A, Printza A, Metaxas S, Constantinidis J (2010) Effects of smoking on taste: assessment with contact endoscopy and taste strips. Laryngoscope 120:1958–1963
Vennemann MM, Hummel T, Berger K (2008) The association between smoking and smell and taste impairment in the general population. J Neurol 255:1121–1126
Pavlidis P, Nikolaidis V, Anogeianaki A, Koutsonikolas D, Kekes G, Anogianakis G (2009) Evaluation of young smokers and non-smokers with electrogustometry and contact endoscopy. BMC Ear Nose Throat Disord 9:9. doi:10.1186/1472-6815-9-9
Fischer R, Griffin F, Kaplan AR (1963) Taste thresholds, cigarette smoking and food dislikes. J Exp Med 9:151–167
Tomassini S, Cuoghi V, Catalani E, Casini G, Bigiani A (2007) Long-term effects of nicotine on rat fungiform taste buds. Neuroscience 147:803–810
Perkins KA, Scott J (2008) Sex differences in long-term smoking cessation rates due to nicotine patch. Nicot Tob Res 10:1245–1250
Bridges RB, Humble JW, Turbek JA, Rehm SR (1986) Smoking history, cigarette yield and smoking behavior as determinants of smoke exposure. Eur J Respir Dis 146:129–137
Acknowledgments
The authors thank Mr. Stefan Kup, member of the Department of Informatics in Marienhof Hospital (Koblenz, Germany) for his help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pavlidis, P., Gouveris, C., Kekes, G. et al. Changes in electrogustometry thresholds, tongue tip vascularization, density and form of the fungiform papillae in smokers. Eur Arch Otorhinolaryngol 271, 2325–2331 (2014). https://doi.org/10.1007/s00405-014-3003-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-014-3003-9