Abstract
The surrogate markers for subclassifying eosinophilic chronic rhinosinusitis (ECRS) and non-ECRS remain elusive. We herein performed a cross-sectional study to assess the clinical implication of clinical symptoms, CT findings, blood eosinophil (EOS) examination based on histological examination of tissue eosinophilia in 105 adult CRS patients (including 72 with nasal polyps and 33 without nasal polyps) in southern China. We found the mean score of smell loss was significantly higher in ECRS subgroup than those in non-ECRS subgroup (p < 0.05), whereas the average ethmoid osteitis index in non-ECRS subgroup was significantly higher than that in ECRS subgroup (p < 0.05). Moreover, we found both the mean blood EOS number and ratio were significantly higher in ECRS subgroup than those in non-ECRS subgroup (p < 0.05). By applying receiver operating characteristic (ROC) curve analysis, we found blood EOS number had a sensitivity of 84.9 % and specificity of 84.4 % [area under the curve (AUC): 0.873] at the cutoff level of 0.16 × 109/L, and blood EOS ratio had a sensitivity of 89.0 % and specificity of 84.4 % (AUC: 0.863) at the cutoff level of 2.05 % in this cohort. Our findings indicated that smell loss score, ethmoid osteitis index and blood EOS number and ratio may be used for the differential diagnosis of ECRS as the surrogate markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic rhinosinusitis (CRS) is characterized by inflammation of the nasal mucosa and paranasal sinuses of at least 12 weeks duration. Based on the presence or absence of nasal polyps, CRS has been most commonly subdivided into 2 clinical subsets: CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP) [1, 2]. In general, CRSsNP tends to be neutrophilic and CRSwNP is considered eosinophilic. The presence or absence of nasal polyps has been extensively used in routine clinical practice for defining differential therapies in several international consensuses for the diagnosis and management of CRS [2–4].
However, CRS in reality contains a group of disorders with large degree of heterogeneity in the underlying pathophysiology and associated histological features, and both the presence and extent of eosinophilia in CRSwNP can be quite variable in different regions [5]. In the Western population, more than 80 % CRSwNP patients demonstrated significant eosinophilic inflammation. In contrast, in China and other Asian countries, recent studies showed that 30–50 % of CRSwNP patients presented non-eosinophilic inflammation [6–8]. In addition to CRSwNP, significant tissue eosinophilia was also observed in some patients with CRSsNP. For example, Snidvongs et al. [9] reported approximately 20 % of CRSsNP demonstrate significant tissue eosinophilia with increased disease severity. Taken together, the categorization based on simple clinical phenotype (the presence or absence of nasal polyps) has proved difficult to apply for improved prognostication and evidence-based management of the inflammation condition.
As an alternative, endotyping CRS patients with histopathological classifications [eosinophilic CRS (ECRS) versus non-eosinophilic CRS (non-ECRS)], rather than simple clinical phenotypes (CRSwNP versus CRSsNP), may represent a more appropriate approach to individualized disease management [10]. Currently, the criterion and surrogate markers for subclassifying ECRS and non-ECRS remain elusive. To address this issue, we performed a cross-sectional study to assess the diagnostic values of clinical parameters [clinical symptoms, CT findings, blood examination of eosinophil (EOS) for ECRS] based on histological examination of tissue eosinophilia in southern China. Our findings may be of interest for improved differential diagnosis and individualized management for CRS patients.
Materials and methods
Subjects
A cross-sectional study of consecutive patients undergoing sinus surgery was undertaken. One hundred and five adult CRS patients (>18 years) (including 72 CRSwNP and 33 CRSsNP) who were ready for endoscopic sinus surgery in our hospital were recruited. The diagnosis of CRS was in accordance with the criterion set by the EPOS 2012 position paper [2]. The atopic status of CRS patients was evaluated by skin prick test or using the ImmunoCAP test (Phadia, Uppsala, Sweden) for detecting IgE antibodies against various common inhalant allergens. All patients were ready sinus surgery for after failing previous medical therapy. No patients were using oral steroid for 4 weeks prior to surgery.
Data from clinical presentations (symptom severity was scored using a 7-point visual analog scale) [4], histopathology [hematoxylin and eosin (HE) stain], computed tomography (CT) scan and blood markers (EOS number and ratio) were collected and analyzed. The demographic characteristics are listed in Table 1. The study had ethical approval from the institutional review board of the First Affiliated Hospital of Sun Yat-sen University. All participants provided their written informed consent to participate in this study.
Histological examination
In the histological study, standard HE stain was applied on all sinus tissues. The sections were examined and scored by two independent observers who were blind to the clinical data. The number of EOS was counted in 10 randomly selected high power fields (HPFs) (400× magnification) and averaged. CRS was classified as eosinophilic when the average number of tissue EOS was more than 5/HPFs (magnification, ×400), as suggested by Kountakis et al. [11].
Radiological assessment
All preoperative CT scans (within 4 weeks) were evaluated with Lund-Mackay score and radiological osteitis index. The maximum bone thickness of the ethmoid and maxillary sinuses was measured, and the sphenoid and frontal sinus thickness was not evaluated. The thickening bony wall or partition was defined as bone thickness >3 mm radiologically. A system using CT to diagnosis osteitis based on the thickness of bony partitions in the ethmoid sinus was described by Lee et al. [12]. The ethmoid osteitis index was scored as follows: 1, mild, bony partition is <3 mm; 2, moderate, bony partition is between 3 and 5 mm; 3, severe, bony partition is more than 5 mm.
Blood parameters
A complete peripheral blood cell count with differential calculation was performed by automated analysis, and the blood EOS number and ratio were recorded.
Statistical analysis
The number and ratio of tissue and blood EOS were expressed as medians and interquartile ranges (IQRs) and analyzed using a nonparametric Mann–Whitney U test. Some continuous variables are expressed as means and standard deviations (SD) of the mean and analyzed using a Student’s t test. Chi-square analysis or Fisher’s exact test was used for relationships of nominal variables. The Spearman rank correlation test was used to analyze the correlation among different parameters. A receiver operating characteristic (ROC) curve was generated to find the best cutoff of a predictor for the diagnosis of ECRS. The area under the curve (AUC) for each potential predictor was calculated; an AUC with a value close to 1 indicates an excellent ability to discriminate. A p value <0.05 was considered statistically significant.
Results
Patient demographics
In this cross-sectional study, we consecutively enrolled 105 CRS patients (including 59 male and 46 female) for analysis. The average age was 33.4 ± 15.2 years old, and the mean disease duration was 7.7 ± 6.7 years. Of them, 30 patients (28.5 %) were smokers, 44 patients (41.9 %) were atopic and 8 patients (7.6 %) had a history of asthma, 72 patients (68.5 %) were with nasal polyps (CRSwNP), 45 patients (42.8 %) had a history of sinus surgery. The major and minor clinical symptoms included nasal congestion (81.9 %), anterior nasal rhinorrhea (73.3 %), posterior nasal rhinorrhea (61.9 %), facial pain/fullness (27.6 %), headache (58.1 %), fatigue (75.2 %), smell loss (66.7 %), ear pain/fullness (38.1 %), cough (41.9 %), halitosis (50.5 %), dental pain (23.8 %) and fever (13.3 %). Based on the histological criteria (tissue EOS number >5/HPFs) of ECRS suggested by Kountakis et al. [11], we identified 73 ECRS patients (69.5 %) and 32 non-ECRS patients (30.5 %) in this cohort.
Symptom parameter
As shown in Table 1, we found the ratio of concurrent nasal polyps, asthma and atopy was significantly higher in ECRS subgroup than those in non-ECRS subgroup (p < 0.05). Of the 12 main and minor symptoms documented (Table 2), we only found the mean score of smell loss was significantly higher in ECRS subgroup than those in non-ECRS subgroup (p < 0.05). Other symptoms, such as nasal obstruction, anterior nasal rhinorrhea, posterior nasal rhinorrhea, facial pain/fullness, headache, fatigue, ear pain/fullness, cough, halitosis, dental pain and fever, exclusively showed no significant difference in 2 subgroups. These findings suggested that smell loss may be used as an indicator for ECRS patients.
CT parameter
The representative findings of ECRS and non-ECRS determined by histological examination and CT scan were shown in Fig. 1. Both ECRS and non-ECRS exerted extensive involvement and enhanced sinus pacification in the advanced stage (disease duration >5 years). As illustrated in Table 3, we found the Lund-Mackay scores of nasal sinuses (maxillary sinus, anterior ethmoid sinus, posterior ethmoid sinus, frontal sinus and sphenoid sinus) and ostiomeatal complex showed no significant difference in 2 subgroups. However, when specifically evaluating the bone thickening in the maxillary sinus and ethmoid sinus in CRS patients, we found that thickening bony wall and partition in the maxillary or ethmoid sinus was more common in non-ECRS subgroup than in ECRS subgroup (27/32 versus 18/73)(p < 0.01). When an osteitis index was applied for evaluating the thickening of bony partition in ethmoid sinus, we found the average ethmoid osteitis index in ECRS subgroup was significantly lower than that in non-ECRS subgroup (1.26 ± 0.50 versus 2.72 ± 0.52, p < 0.01). These findings suggested that ethmoid osteitis index, rather than Lund-Mackay score, may be used as a counter indicator for ECRS patients.
Blood parameters
To further investigate the surrogate markers for ECRS patients, we next examined the blood parameters in this cohort. In these 105 CRS patients, the mean blood EOS number and ratio were 0.26 ± 0.19 × 109/L, and 3.34 ± 2.37 %, respectively. Both the mean blood EOS number and ratio were significantly higher than those in the normal controls (Fig. 2, p < 0.05). When the mean blood EOS number and ratio with tissue EOS number in CRS patients were correlated, we found the mean blood EOS number, as well as blood EOS ratio, was positively related to the tissue EOS number, with significant difference (Fig. 3, p < 0.05). Moreover, when the CRS patients were subdivided into ECRS and non-ECRS subgroups, we found the mean blood EOS number in ECRS and non-subgroups were 0.34 (0.22, 0.40) × 109/L and 0.07 (0.04, 0.12) × 109/L, respectively, and the mean blood EOS ratio in ECRS and non-ECRS subgroups were 3.80 (2.80, 5.30) % and 1.25 (0.40, 1.90) %, respectively. Both the mean blood EOS number and ratio were significantly higher in ECRS subgroup than those in non-ECRS subgroup (p < 0.01).
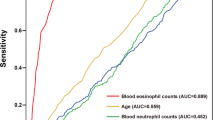
To further validate the clinical implication of mean blood EOS number and ratio in the differential diagnosis of ECRS and non-ECRS, we next performed ROC curve analysis of mean blood EOS number and ratio in ECRS and non-ECRS patients. As illustrated in Fig. 4, we found blood EOS number had a sensitivity of 84.9 % and specificity of 84.4 % (AUC: 0.873) at the cutoff level of 0.16 × 109/L, and blood EOS ratio had a sensitivity of 89.0 % and specificity of 84.4 % (AUC: 0.863) at the cutoff level of 2.05 %. These findings suggested that the mean blood EOS number, as well as blood EOS ratio, may be used as a useful surrogate marker for the diagnosis of ECRS as well.
Discussion
In the present study, we have shown that the mean score of smell loss was significantly higher in ECRS subgroup than those in non-ECRS subgroup, whereas the average ethmoid osteitis index in non-ECRS subgroup was significantly higher than that in ECRS subgroup. Moreover, we found both the mean blood EOS number and ratio were significantly higher in ECRS subgroup than those in non-ECRS subgroup. Therefore, our finding may be beneficial to the differential diagnosis and subsequent personalized therapy of ECRS and non-ECRS patients.
Recent study showed that the inflammatory patterns in sinus disease in the same geographical area may change toward eosinophilic phenotype over time, suggesting that the inflammatory patterns of ECRS and non-ECRS may not only vary with the location, but also over time [5, 13]. Moreover, it has been proposed that ECRS patients have differential disease severity and treatment outcomes compared to non-ECRS patients [14]. Thus, differential diagnosis of ECRS and non-ECRS patients may represent the first logistical step to establish individualized management [15]. Currently, there is no official gold standard diagnostic test for ECRS. Histology is considered the most accurate method, and most histological criteria which have been proposed are based on tissue EOS number. For example, in Kountakis et al. [11] study, they proposed a cutoff value for non-ECRS by evaluating the degree of sinus tissue eosinophilia (≤5 EOS/HPFs). Consequently, they found that ECRS patients had higher frequency of nasal polyps and asthma and higher CT and endoscopy scores than non-ECRS patients. In the present study, we firstly examined the clinical characteristics of ECRS and non-ECRS patients. The ECRS patients had been identified by adopting a histological criterion proposed by Kountakis et al. As a result, we identified 73 ECRS patients (69.5 %) and 32 non-ECRS patients (30.5 %) in this cohort. In agreement with Ouyang’s report, we found the mean score of smell loss was significantly higher in ECRS subgroup than those in non-ECRS subgroup (p < 0.05). These findings suggested that smell loss may be used as an indicator for ECRS patients. Our findings were in contrast to the previous report that clinical characteristics of ECRS are apparently different from that of non-ECRS [14], which may be ascribed to the advanced stage of CRS patients included in this cohort.
Although the diagnosis of ECRS has prognostic implications, it is not a routine to conduct histological examination in CRS patients before sinus surgery. Alternatively, radiological findings such as CT scan may be used as a surrogate marker for ECRS patients. For example, Ishitoya et al. proposed that ECRS patients showed ethmoid predominance in early stages, whereas non-ECRS patients showed maxillary predominance in early stages under CT scan. Based on these findings, Sakuma et al. have established a set of CT parameters (olfactory cleft score >1 and posterior ethmoid score >1) in addition to blood examination to differentiate ECRS from non-ECRS in regular outpatient clinics [16]. In this study, however, we found both ECRS and non-ECRS exerted extensive involvement and enhanced sinus pacification, and the Lund-Mackay scores of nasal sinuses and ostiomeatal complex showed no significant difference in 2 subgroups. This may be ascribed to the advanced stage of CRS patients included in this cohort. However, when specifically evaluating the bone thickening in the maxillary sinus and ethmoid sinus in CRS patients, we found that thickening bony wall and partition in the maxillary or ethmoid sinus were more common in non-ECRS subgroup than in ECRS subgroup (27/32 versus 18/73), which was contrast to Snidvongs et al.’s [17] recent report that CRS patients with mucosal eosinophilia had higher osteitis score than those without. Moreover, when an osteitis index was applied for evaluating the thickening of bony partition in ethmoid sinus, we found the average ethmoid osteitis index in ECRS subgroup was significantly lower than that in non-ECRS subgroup. These findings suggested that ethmoid osteitis index, rather than Lund-Mackay score, may be used as a counter indicator for ECRS patients.
CRSwNP is generally characterized by Th2-skewed inflammation and enhanced tissue eosinophilia in western population [18]. Although the data are compelling that eosinophilia is more likely to be associated with the presence of nasal polyps, both the presence and extent of eosinophilia in nasal polyps can be quite variable [18, 19], and a large subset of nasal polyps observed in CRSwNP patients in China, Japan or other countries in East Asia show non-eosinophilic inflammation [6–8]. On the other hand, enhanced tissue eosinophilia is also present in up to 19 % of CRSsNP patients [9]. These findings suggested that nasal polyps itself cannot be considered a convincing indicator of tissue eosinophilia or ECRS. In some studies, tissue and blood eosinophilia showed significant correlations in CRS patients, providing the possibility that blood EOS can be used as the surrogate marker of ECRS patients [16]. Consistently, in this cohort, we found both the mean blood EOS number and ratio were significantly higher than those in the normal controls. When the mean blood EOS number and ratio with tissue EOS number in CRS patients were correlated, we found the mean blood EOS number, as well as blood EOS ratio, was positively related to the tissue EOS number. Moreover, we found both the mean blood EOS number and ratio were significantly higher in ECRS subgroup than those in non-ECRS subgroup. These findings suggested blood EOS parameter may be used as a simpler and more practical strategy for subclassification of ECRS and non-ECRS.
Some authors recently attempted to examine blood EOS number and ratio to identify the diagnostic accuracy for ECRS patients. For example, Sakuma et al. proposed a blood parameter (increased blood EOS percentage above the normal range) in addition to clinical and CT examination to differentiate ECRS from non-ECRS and obtained high accuracy (sensitivity, 84.6 %; specificity, 92.3 %) in regular outpatient clinics, but they failed to obtain histological confirmation [16]. Recently, Hu et al. enrolled 155 CRSwNP patients in a cohort in central China and examined the diagnostic significance of blood EOS count for eosinophilic CRSwNP [20]. They found an absolute blood EOS count of 0.215 × 109/L yielded a sensitivity of 74.2 % and a specificity of 86.5 %, and a blood EOS percentage of 3.05 % yielded a sensitivity of 80.3 % and a specificity of 75.3 % for the diagnosis of eosinophilic CRSwNP. However, they specifically defined ECRS using tissue EOS ratio as tissue criterion of eosinophilic CRSwNP, which may not be as same convincing as tissue EOS number. In the present study, we firstly conducted the differential diagnosis of ECRS and non-ECRS based on a well-recognized histological criterion (tissue EOS number >5/HPFs), which makes the subsequent findings more reliable and scientific. Similar to the aforementioned findings, we found blood EOS number had a sensitivity of 84.9 % and specificity of 84.4 % (AUC: 0.873) at the cutoff level of 0.16 × 109/L, and blood EOS ratio had a sensitivity of 89.0 % and specificity of 84.4 % (AUC: 0.863) at the cutoff level of 2.05 % for the diagnosis of ECRS in this cohort. Collectively, our findings suggested that the mean blood EOS number, as well as blood EOS ratio, may be used as a useful surrogate marker for the diagnosis of ECRS in southern China.
Conclusion
We found smell loss score, ethmoid osteitis index and blood EOS number and ratio may be used for the differential diagnosis of ECRS. Further study is required to comparably examine the differential responsiveness of ECRS and non-ECRS patients to steroid and macrolide treatment.
References
Hamilos DL (2011) Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol 128:693–707
Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern B, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskala E, Voegels R, de Wang Y, Wormald PJ (2012) EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50:1–12
Marple BF, Stankiewicz JA, Baroody FM, Chow JM, Conley DB, Corey JP, Ferguson BJ, Kern RC, Lusk RP, Naclerio RM, Orlandi RR, Parker MJ, American Academy of Otolaryngic Allergy Working Group on Chronic Rhinosinusitis (2009) Diagnosis and management of chronic rhinosinusitis in adults. Postgrad Med 121:121–139
Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Bachert C, Baraniuk J, Baroody FM, Benninger MS, Brook I, Chowdhury BA, Druce HM, Durham S, Ferguson B, Gwaltney JM, Kaliner M, Kennedy DW, Lund V, Naclerio R, Pawankar R, Piccirillo JF, Rohane P, Simon R, Slavin RG, Togias A, Wald ER, Zinreich SJ, American Academy of Allergy, Asthma and Immunology (AAAAI), American Academy of Otolaryngic Allergy (AAOA), American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS), American College of Allergy, Asthma and Immunology (ACAAI), American Rhinologic Society (ARS) (2004) Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 114(6 Suppl):155–212
Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C (2011) Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol 128:728–732
Katotomichelakis M, Tantilipikorn P, Holtappels G, De Ruyck N, Feng L, Van Zele T, Muangsomboon S, Jareonchasri P, Bunnag C, Danielides V, Cuvelier CA, Hellings PW, Bachert C, Zhang N (2013) Inflammatory patterns in upper airway disease in the same geographical area may change over time. Am J Rhinol Allergy 27:354–360
Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS (2007) Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg 137:925–930
Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, Wang DY, Desrosiers M, Liu Z (2009) Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol 124:478–484 (pp 484.e1–2)
Snidvongs K, Lam M, Sacks R, Earls P, Kalish L, Phillips PS, Pratt E, Harvey RJ (2012) Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int Forum Allergy Rhinol 2:376–385
Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, Schleimer RP, Ledford D (2013) Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 131:1479–1490
Kountakis SE, Arango P, Bradley D, Wade ZK, Borish L (2004) Molecular and cellular staging for the severity of chronic rhinosinusitis. Laryngoscope 114:1895–1905
Lee JT, Kennedy DW, Palmer JN, Feldman M, Chiu AG (2006) The incidence of concurrent osteitis in patients with chronic rhinosinusitis: a clinicopathological study. Am J Rhinol 20:278–282
Kim SJ, Lee KH, Kim SW, Cho JS, Park YK, Shin SY (2013) Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol Head Neck Surg 149:431–437
Ishitoya J, Sakuma Y, Tsukuda M (2010) Eosinophilic chronic rhinosinusitis in Japan. Allergol Int 59:239–245
Chin D, Harvey RJ (2013) Nasal polyposis: an inflammatory condition requiring effective anti-inflammatory treatment. Curr Opin Otolaryngol Head Neck Surg 21:23–30
Sakuma Y, Ishitoya J, Komatsu M, Shiono O, Hirama M, Yamashita Y, Kaneko T, Morita S, Tsukuda M (2011) New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus Larynx 38:583–588
Snidvongs K, McLachlan R, Chin D, Pratt E, Sacks R, Earls P, Harvey RJ (2012) Osteitic bone: a surrogate marker of eosinophilia in chronic rhinosinusitis. Rhinology 50:299–305
Hsu J, Peters AT (2011) Pathophysiology of chronic rhinosinusitis with nasal polyp. Am J Rhinol Allergy 25:285–290
Payne SC, Borish L, Steinke JW (2011) QueryGenetics and phenotyping in chronic sinusitis. J Allergy Clin Immunol 128:710–720
Hu Y, Cao PP, Liang GT, Cui YH, Liu Z (2012) Diagnostic significance of blood eosinophil count in eosinophilic chronic rhinosinusitis with nasal polyps in Chinese adults. Laryngoscope 122:498–503
Acknowledgments
This study was supported by the National Natural Science Fund of China (No. 81070771, 81070772, 81271054) and a grant from the Ministry of Hygiene (No. 201202005) and Program for New Century Excellent Talents in University (No. NCET-10-0851).
Conflict of interest
No conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Zuo and J. Guo have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zuo, K., Guo, J., Chen, F. et al. Clinical characteristics and surrogate markers of eosinophilic chronic rhinosinusitis in Southern China. Eur Arch Otorhinolaryngol 271, 2461–2468 (2014). https://doi.org/10.1007/s00405-014-2910-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-014-2910-0