Abstract
Sudden sensorineural hearing loss is usually treated with systemic glucocorticoids. Intratympanic injections of glucocorticoids offer a possibly equivalent treatment alternative, avoiding adverse systemic effects on blood glucose. We, therefore, investigated the extent to which different doses of systemic glucocorticoid therapy affects blood glucose levels. We conducted a retrospective analysis of treatment courses in 179 patients from the Departments of Otorhinolaryngology, Ophthalmology and Dermatology who underwent short-course systemic glucocorticoid therapy. Patients were subdivided into three groups on the basis of their cumulative prednisolone dose from days 1 to 3 (Group 1: <750 mg; Group 2: 750–1,499 mg; Group 3: >1,499 mg); in addition, a distinction was made between diabetic and non-diabetic patients. Among the non-diabetic patients on days 2–4, diabetic levels of fasting blood glucose were detected significantly more often (P < 0.01) in Group 3 (67 %) than in Group 1 (28 %) and Group 2 (21 %). Furthermore, there was a highly significant mean Pearson correlation (r = 0.329; P < 0.01) between blood glucose levels and glucocorticoid dose. This correlation was even more pronounced in the diabetic patients (r = 0.51; P = 0.02). In this category, hyperglycemia was detected in 40 % of patients in Group 1, 63 % in Group 2 and 100 % in Group 3. The prevalence of glucocorticoid-induced hyperglycemia during systemic therapy is high and rises as the dose increases. This should be kept in mind when choosing the dosage. Besides, it should also be considered that even short-term hyperglycemia presents possible health risks and the risk of inducing diabetes. This is especially of interest as intratympanic therapy offers a possible alternative to the systemic application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sudden sensorineural hearing loss (SSHL), first described by De Kleyn in 1944, is almost invariably characterized by partial or complete unilateral hearing loss, often associated with tinnitus and vertigo [1]. According to the classic definition, SSHL develops within a period of not more than 72 h and affects at least three sequential frequencies, with hearing loss of at least 30 dB in the pure-tone audiogram [2, 3]. The annual incidence is between 5 and 30 per 100,000 population. However, a large number of cases presumably go unreported, because many patients who experience rapid spontaneous recovery elude medical care [1, 3]. The average age at initial presentation is 50–60 years with equal distribution between men and women [1]. Probably because the pathophysiology of SSHL is still largely unelucidated, its treatment is far from the standard and ranges from simple observation through to the concurrent use of multiple medicinal products [4, 5]. Systemic glucocorticoids have been used in the management of SSHL for more than 30 years from now, and even today, they remain the most accepted and commonly recommended treatment modality [3, 4, 6]. Overall, glucocorticoids have a wide spectrum of indications, especially in the treatment of chronic inflammatory and autoimmune diseases [7]. However, a careful watch must be kept for pronounced adverse systemic effects such as central adiposity, dyslipidemia, loss of muscle mass, and hyperglycemia through to diabetes mellitus [8]. Glucocorticoids are, therefore, the commonest cause of pharmacologically induced diabetes [9]. According to clinical observation studies, the prevalence of new-onset diabetes in the context of glucocorticoid therapy is as high as 46 % [10]. The raised blood glucose levels in this setting are caused both by reduced insulin production by the ß-cells of the pancreas and by increased insulin resistance [11]. The published literature primarily contains reports of the association between hyperglycemia and high-dose long-term glucocorticoid therapy, e.g., following organ transplantation or in the treatment of rheumatoid arthritis. In contrast, little research has been conducted to date into the effect of short-term therapy (<4 weeks) with a variety of dosage levels, as implemented in the management of SSHL. Since intratympanic glucocorticoid injections are a possible alternative option in the treatment of SSHL, potentially allowing adverse systemic effects to be avoided, the present study was conducted to investigate whether and to what extent systemic glucocorticoid therapy causes hyperglycemia. With this objective in mind, different dosages of systemic glucocorticoid therapy were analyzed for their effect on blood glucose levels in patients with and without diabetes.
Subjects and methods
The study was approved by the Ethics Committee of the University.
Patient population and data collection
To obtain a representative patient population for the present study, treatment details were examined retrospectively for all patients who received systemic glucocorticoids during the period from January 2008 to December 2009 in the Departments of Otorhinolaryngology (ORL), Ophthalmology and Dermatology. The principal underlying conditions were SSHL, vestibular dysfunction and Menière’s disease in ORL, alopecia areata, allergic urticaria and pyoderma gangrenosum in Dermatology, and giant cell arteritis in Ophthalmology (Table 1). Studying different clinical conditions across three medical specialties permitted the effect of different glucocorticoid dose levels to be analyzed. Therefore, even the effects of very high cortisone dosages (>1,499 mg) like they are used for the initial shock therapy of giant cell arteritis with threatening blindness could be examined. All glucocorticoid preparations used were converted to prednisolone equivalents. The inclusion criterion was a minimum daily dose above the Cushing threshold of 7.5 mg. Patients were excluded from the study if their glucocorticoid dose was lower than this value or if blood glucose was not documented in the course of glucocorticoid therapy.
Alongside blood glucose levels and details of the glucocorticoid dose, information was collected from the patients’ records concerning age, sex, body mass index (BMI), previous illnesses, medication, length of hospitalization, and underlying illness.
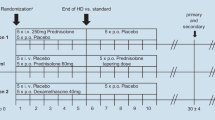
For the subsequent analysis, the study population was subdivided into patients with and without a previous diagnosis of diabetes mellitus (ascertained by anamnesis, medical records and medication). To ensure a clear differentiation between these two groups, three patients with latent diabetes (temporarily increased blood sugar levels in the past) were excluded from the study. In addition, groups were formed on the basis of the cumulative 3-day glucocorticoid dose: <750 mg (Group 1), 750–1,499 mg (Group 2) and >1,499 mg (Group 3). All patients suffering SSHL were treated over 5 days with descending prednisolone doses of 500, 250, 250, 250, 100 mg, respectively.
Statistics
The statistical analysis was performed using the PASW 18.0 program (SPSS Inc., Chicago, USA). The primary analysis explored the potential correlation between fasting and postprandial blood glucose values on the one hand and glucocorticoid therapy and dosage level on the other. Possible correlations were also sought for patients’ sex, age, previous illnesses, medication and body weight. Descriptive statistics were used for the analysis of blood glucose values, glucocorticoid dosage, diabetic versus non-diabetic, main diagnoses, age, sex, length of hospitalization, and BMI. The Pearson correlation coefficient was used to calculate the association between the glucocorticoid dose on days 1–3 and the fasting and postprandial blood glucose levels on days 2–4. A possible association with age or BMI was also explored using the Pearson correlation coefficient. Possible influences of sex, previous illnesses and medication were studied using the Chi-squared test.
Results
Patient population
Altogether, 179 patients (mean age: 54.3 years) were included in our study. Of this number, 102 patients were female and 77 male. One hundred patients had been treated in Otorhinolaryngology, 58 in Dermatology and 21 in Ophthalmology. The commonest underlying condition was SSHL in a total of 25.7 % of cases. The mean duration of hospitalization was 6.5 days. No previous diabetes was known in 87.7 % of the patients (n = 157); the remaining 22 patients had diabetes, and 5 of them were insulin-dependent. Because of the important benefit of a successful therapy for the patients (such as recovery of hearing or preservation of vision), previously known diabetics were also treated with systemic glucocorticoids. The potential risk of hyperglycemia was counterbalanced with diabetes medication adapted to controls of blood glucose levels. The mean BMI was 26.6 (range: 10.0–44.8). According to the WHO classification of BMI, 32.4 % of the patients were in the normal range, 23.5 % were pre-obese, 20.7 % were obese (obesity class I–III), and one woman was underweight.
Blood glucose levels
On the basis of the cumulative glucocorticoid dose on days 1–3, 42 patients (30 non-diabetic, 12 diabetic) were assigned to Group 1 (prednisolone dose <750 mg), 95 patients (87 non-diabetic, 8 diabetic) to Group 2 (750–1,499 mg), and 42 patients (40 non-diabetic, 2 diabetic) to Group 3 (>1,499 mg). The reason for the evaluation of the cumulative glucocorticoid dose of days 1–3 was a considerable reduction of the mean glucocorticoid dose afterwards. Assuming a certain time delay of a possible effect on the blood glucose, we consequently evaluated blood glucose levels of days 2–4. The level of the glucocorticoid dose on days 1–6 in each group is shown in Fig. 1. Figure 2 shows the blood glucose curves of the individual groups for days 2–7. Blood glucose levels were assessed using the criteria for the diagnosis of diabetes mellitus issued by the American Diabetes Association in 2004 [12]. According to the ADA position statement, a fasting plasma glucose level <100 mg/dl is considered as normal fasting glucose, a level between 100 and 125 mg/dl as impaired fasting glucose, and a level ≥126 mg/dl as diabetes. The postprandial blood glucose levels were assessed according to the 2 h diagnostic values for the oral glucose tolerance test. Accordingly, postprandial levels <140 mg/dl were classified as normal glucose tolerance, 140–199 mg/dl as impaired glucose tolerance and ≥200 mg/dl as diabetes [12]. Using the criteria set out in these guidelines, the mean fasting blood glucose levels on days 2–4 during glucocorticoid therapy in the non-diabetic patients were classified as ‘impaired fasting glucose’ in 40 % of patients in Group 1, in 52 % of those in Group 2 and in 28 % of those in Group 3. The criteria for a diagnosis of diabetes were fulfilled in 28 % of patients in Group 1, in 21 % of those in Group 2 and in 67 % of those in Group 3 (Fig. 3). Among the diabetic patients a fasting blood glucose level ≥126 mg/dl was found in 40 % of patients in Group 1, in 63 % of those in Group 2, and in 100 % of those in Group 3 (Fig. 4). In the non-diabetic patients, assessment of the correlation between the mean fasting blood glucose levels on days 2–4 and the cumulative glucocorticoid dose on days 1–3 revealed a highly significant (P < 0.01) mean correlation, with a Pearson correlation coefficient of r = 0.329. In the diabetic patients, there was a significant high correlation (P = 0.02), with a Pearson correlation coefficient of r = 0.51. No correlation was detected between glucocorticoids and postprandial blood glucose levels (P = 0.485, Pearson correlation coefficient r = 0.092). Similarly, no significant correlation was found between blood glucose levels and sex (P = 0.485), age (P = 0.145), BMI (P = 0.109) and the patients’ various previous illnesses and medications.
Blood glucose in non-diabetic patients. Percentage distribution of mean fasting blood glucose on days 2–4 in patients with no previous diagnosis of diabetes (n = 157) in Group 1 (<750 mg), Group 2 (750–1,499 mg) and Group 3 (>1,499 mg): blue normal fasting glucose (<100 mg/dl), red impaired fasting glucose (100–125 mg/dl), and green diabetes (≥126 mg/dl)
Blood glucose in diabetic patients. Percentage distribution of mean fasting blood glucose on days 2–4 in patients with previous diagnosis of diabetes (n = 22) in Group 1 (<750 mg), Group 2 (750 –1,499 mg) and Group 3 (>1,499 mg): blue normal fasting glucose (<100 mg/dl), red impaired fasting glucose (100–125 mg/dl), and green diabetes (≥126 mg/dl)
Discussion
Systemic glucocorticoids are one of the standard treatment modalities for SSHL. Back in 1980 Wilson et al. [13] showed in a double-blind controlled study that patients receiving systemic glucocorticoid therapy recovered their hearing significantly more often than patients receiving placebo (61 % vs. 32 %). In another prospective study, Moskowitz et al. [14] reported recovery of hearing in 89 % of patients receiving glucocorticoid therapy compared with 44 % of controls. Currently, intravenous administration of 250 mg prednisolone over 3 days is also recommended, in line with guidelines issued by the German ENT Society [4]. These guidelines also form the basis for the SSHL treatment regimen used in our study, according to which patients are treated over 5 days with descending prednisolone doses of 500, 250, 250, 250, 100 mg, respectively. Furthermore, according to a prospective study by Niedermeyer et al. [15], a dose of at least 250 mg appears to be advisable. In 29 patients who had undergone stapedectomy, these authors evaluated the levels of cortisol in the perilymph of the labyrinth after intravenous administration of different doses of prednisolone. Whereas significantly elevated perilymphatic cortisol levels were detected after an injection of 250 mg prednisolone, a dose of only 125 mg did not result in significantly raised levels compared with the control group.
The dosage and duration of use of glucocorticoids are limited principally by the extensive metabolic side-effect profile of these substances [8]. Clinical studies have shown that systemic glucocorticoids may not only increase the risk of hyperglycemia and induce diabetes in non-diabetic patients but may also complicate the regulation of blood glucose in diabetic patients [11]. To date, however, it is largely unclear at what dosage level or after how long these adverse effects are likely to occur.
In our study, in all three groups receiving different glucocorticoid doses, there was a close correlation with raised fasting blood glucose levels. However, depending on the dose used, the effects occurred with differing frequency: a prednisolone dose >1,499 mg (Group 3) resulted in diabetic fasting blood glucose levels significantly more often (P < 0.01) than doses <1,500 mg (Groups 1 and 2). At this high dose, diabetic hyperglycemia occurred in 67 % of non-diabetic patients and in all diabetic patients. However, hyperglycemia was also commonly noted at lower doses. In SSHL patients, for example, diabetic fasting blood glucose levels occurred in 21 % of non-diabetics and in 63 % of diabetics in Group 2 (750–1,499 mg), and in 28 % of non-diabetics and 40 % of diabetics in Group 1 (<750 mg). No significant difference was found in this respect between Group 1 and Group 2 (P = 0.571). A lower dose, i.e., <750 mg, therefore, conferred no benefit in terms of glucose metabolism compared with 750–1,499 mg, whereas doses >1,499 mg caused hyperglycemia significantly more frequently. The extremely high overall prevalence of hyperglycemia in response to cortisone therapy in our study is in agreement with the results of similar studies in the literature. For example, Donihi et al. [10] in a retrospective study detected hyperglycemia ≥200 mg/dl in 64 % of patients who received a prednisolone dose ≥40 mg/day for at least 2 days. It is of major importance that these instances of hyperglycemia are recognized, and where necessary, treated. Firstly, not only chronic but also short-term hyperglycemia is associated with an increased risk of infections, thromboses, endothelial dysfunction, deteriorating cardiovascular status, and major neuronal damage following ischemic stroke [16]. Secondly, glucocorticoids may lead to a ketoacidotic or hyperglycemic coma. At least 60 instances of this phenomenon (some with fatal outcome) have been reported in the literature [10, 17–19]. Furthermore, studies have shown that systemic glucocorticoids more than double the risk of developing persistent diabetes that requires treatment [20, 21]. In this context, a case–control study by Gurwitz et al. [21] showed a clear dependence on the glucocorticoid dose used. Whereas the odds ratio for the development of diabetes requiring treatment following therapy with ≤39 mg/day hydrocortisone was 1.77, it increased to 10.34 at a dose of 120 mg/day. Likewise, gestational diabetes occurred significantly more frequently when cortisone was administered during pregnancy compared with the control group (23.8 vs. 4 %, P = 0.001) [22]. Earlier studies suggest that patients with previously diagnosed diabetes, multiple previous illnesses, advanced age and high BMI are particularly at risk of developing hyperglycemia due to cortisone therapy [10, 23]. However, our study revealed no correlation with these factors.
In view of our study results and given the high prevalence of hyperglycemia and its possible sequelae, we therefore recommend that blood glucose levels should be monitored regularly in all patients receiving systemic glucocorticoid therapy. Reports in the literature suggest that the determination of postprandial blood glucose is particularly sensitive for this purpose [7, 9]. In our study, however, no correlation was found between glucocorticoid dose and postprandial glucose levels. This may have been due to the fact that the postprandial blood glucose levels in our patients were measured in the afternoon and evening. Prednisolone attains peak concentrations 4–6 h after intravenous injection and has a total duration of action of 12–16 h [9, 24]. To mimic the natural time course of cortisone levels, which reach their peak between 06:00 and 08:00 a.m., glucocorticoids are generally given in the morning. In a prospective study in 20 patients, Yuen et al. [7] found that after morning administration of 20 mg prednisone, blood glucose levels were increased principally from midday onward, whereas effects on blood glucose concentrations were no longer detectable at midnight. It is, therefore, possible that glucocorticoid activity and its effect on blood glucose levels had already subsided again by the time of the postprandial measurements in our study. By contrast, fasting blood glucose levels showed a strong correlation with glucocorticoid administration. However, the published literature points out that some patients with glucocorticoid-induced hyperglycemia fail to be identified on the basis of fasting blood glucose levels [23]. In a study by Uzu et al. [25], for example, diabetic postprandial blood glucose values occurred after glucocorticoid therapy (prednisolone: 0.75 mg/kg/day) in 40.5 % of non-diabetic patients, whereas normal fasting blood glucose levels had been measured in all these patients. In another study, diabetic postprandial blood glucose values ≥200 mg/dl were recorded in 50 % of patients receiving prednisolone therapy (ca. 42 mg/day) despite normal fasting blood glucose levels [23]. It is, therefore, possible that in our study not all patients affected were identified and consequently there may have been an even higher prevalence of glucocorticoid-induced hyperglycemia. In the light of currently available studies, therefore, postprandial midday blood glucose should be determined in addition to fasting blood glucose levels.
No clear guidelines are currently available concerning the treatment of glucocorticoid-induced hyperglycemia. The literature contains recommendations in favor of all measures that are also used in type 2 diabetes, e.g., dietary modification and exercise, various oral antidiabetic drugs and insulin [9, 23]. For example, Clore and Thurby-Hay [23] recommend preventing hyperglycemia through the use of NPH insulins (delayed-action insulins), which have an activity profile that is similar to that of prednisolone. Those authors have proposed a treatment regimen with these delayed-action insulins, according to which a morning dose of 0.4 U/kg body weight should be given as a preventive measure when the prednisolone dosage is ≥40 mg/day. In diabetic patients who are already insulin-dependent, this preventive morning dose should be given in addition. Overall, however, the picture presented by studies on the treatment of hyperglycemia induced by cortisone therapy is far from clear. Further investigations are therefore needed to determine which therapeutic measures are required for the management of glucocorticoid-induced hyperglycemia and at what point in time they should be implemented.
One possible alternative to systemic glucocorticoid therapy that has attracted increasing attention in recent years is the intratympanic injection of glucocorticoids. This local form of administration has the potential to avoid the adverse systemic effects of glucocorticoids. One current review article reports the equivalence of intratympanic and systemic glucocorticoid administration in the initial therapy of SSHL. However, the authors concede that the results have only a weak evidence base and that there is a paucity of well-executed studies concerning the efficacy of intratympanic glucocorticoid injection [26]. Therefore, current studies do not yet unequivocally justify the recommendation of intratympanic glucocorticoid injection as an adequate alternative to systemic therapy. Further studies are required to investigate the benefit of intratympanic injection in comparison with systemic glucocorticoid therapy.
Conclusion
Systemic glucocorticoids are currently one of the standard therapies for SSHL. However, our study has shown that these agents—depending on the dose used—cause hyperglycemia in a large proportion of patients. Since current studies suggest that intratympanic injections may be equivalent in terms of treatment outcome, the possibility of these adverse systemic effects should be taken into account when formulating future recommendations and guidelines. Standard recommendations on procedures and therapy to be adopted in glucocorticoid-induced hyperglycemia do not exist to date and these should be established in the light of more extensive studies.
References
Schreiber BE, Agrup C, Haskard DO, Luxon LM (2010) Sudden sensorineural hearing loss. Lancet 375:1203–1211. doi:10.1016/S0140-6736(09)62071-7
Alimoglu Y, Inci E, Edizer DT, Ozdilek A, Aslan M (2011) Efficacy comparison of oral steroid, intratympanic steroid, hyperbaric oxygen and oral steroid + hyperbaric oxygen treatments in idiopathic sudden sensorineural hearing loss cases. Eur Arch Otorhinolaryngol 268:1735–1741. doi:10.1007/s00405-011-1563-5
Rauch SD, Halpin CF, Antonelli PJ, Babu S, Carey JP, Gantz BJ, Goebel JA, Hammerschlag PE, Harris JP, Isaacson B, Lee D, Linstrom CJ, Parnes LS, Shi H, Slattery WH, Telian SA, Vrabec JT, Reda DJ (2011) Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA 305:2071–2079. doi:10.1001/jama.2011.679
Suckfüll M (2005) Up to date: therapy of sudden hearing loss. Laryngorhinootologie 84:277–282. doi:10.1055/s-2005-861268 (quiz 283–287)
Coelho DH, Thacker LR, Hsu DW (2011) Variability in the management of idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 145:813–817. doi:10.1177/0194599811412721
Haynes DS, O’Malley M, Cohen S, Watford K, Labadie RF (2007) Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope 117:3–15. doi:10.1097/01.mlg.0000245058.11866.15
Yuen KC, McDaniel PA, Riddle MC (2011) Twenty-four hour profiles of plasma glucose, insulin, C-peptide and free fatty acid in subjects with varying degrees of glucose tolerance following short-term medium-dose prednisone (20 mg/day) treatment: evidence for differing effects on insulin secretion. Clin Endocrinol. doi:10.1111/j.1365-2265.2011.04242.x
van Raalte DH, Ouwens DM, Diamant M (2009) Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest 39:81–93. doi:10.1111/j.1365-2362.2008.02067.x
Lansang MC, Hustak LK (2011) Glucocorticoid-induced diabetes and adrenal suppression: how to detect and manage them. Clevel Clin J Med 78:748–756. doi:10.3949/ccjm.78a.10180
Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA (2006) Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract 12:358–362
Schacke H, Docke W, Asadullah K (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96:23–43
American Diabetes Association (2004) Diagnosis and classification of diabetes mellitus. Diabetes Care 27:5S. doi:10.2337/diacare.27.2007.S5
Wilson WR, Byl FM, Laird N (1980) The efficacy of steroids in the treatment of idiopathic sudden hearing loss: a double-blind clinical study. Arch Otolaryngol 106:772–776
Moskowitz D, Lee KJ, Smith HW (1984) Steroid use in idiopathic sudden sensorineural hearing loss. Laryngoscope 94:664–666
Niedermeyer HP, Zahneisen G, Luppa P, Busch R, Arnold W (2003) Cortisol levels in the human perilymph after intravenous administration of prednisolone. Audiol Neurootol 8:316–321. doi:10.1159/000073516
Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB (2004) Management of diabetes and hyperglycemia in hospitals. Diabetes Care 27:553–591. doi:10.2337/diacare.27.2.553
Braithwaite SS, Barr WG, Rahman A, Quddusi S (1998) Managing diabetes during glucocorticoid therapy: how to avoid metabolic emergencies. Postgrad Med 104:163–166 (171, 175–176)
Cağdaş DN, Paç FA, Cakal E (2008) Glucocorticoid-induced diabetic ketoacidosis in acute rheumatic fever. J Cardiovasc Pharmacol Ther 13:298–300. doi:10.1177/1074248408326609
Bedalov A, Balasubramanyam A (1997) Glucocorticoid-induced ketoacidosis in gestational diabetes: sequela of the acute treatment of preterm labor: a case report. Diabetes Care 20:922–924
Blackburn D, Hux J, Mamdani M (2002) Quantification of the risk of corticosteroid-induced diabetes mellitus among the elderly. J Gen Intern Med 17:717–720
Gurwitz JH, Bohn RL, Glynn RJ, Monane M, Mogun H, Avorn J (1994) Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med 154:97–101
Fisher JE, Smith RS, Lagrandeur R, Lorenz RP (1997) Gestational diabetes mellitus in women receiving beta-adrenergics and corticosteroids for threatened preterm delivery. Obstet Gynecol 90:880–883
Clore J, Thurby-Hay L (2009) Glucocorticoid-induced hyperglycemia. Endocr Pract 15:469–474. doi:10.4158/EP08331.RAR
Magee MH, Blum RA, Lates CD, Jusko WJ (2002) Pharmacokinetic/pharmacodynamic model for prednisolone inhibition of whole blood lymphocyte proliferation. Br J Clin Pharmacol 53:474–484. doi:10.1046/j.1365-2125.2002.01567.x
Uzu T, Harada T, Sakaguchi M, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Koya D, Haneda M, Kashiwagi A, Yamauchi A (2007) Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract 105:c54–c57. doi:10.1159/000097598
Spear SA, Schwartz SR (2011) Intratympanic steroids for sudden sensorineural hearing loss: a systematic review. Otolaryngol Head Neck Surg 145:534–543. doi:10.1177/0194599811419466
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rohrmeier, C., Koemm, N., Babilas, P. et al. Sudden sensorineural hearing loss: systemic steroid therapy and the risk of glucocorticoid-induced hyperglycemia. Eur Arch Otorhinolaryngol 270, 1255–1261 (2013). https://doi.org/10.1007/s00405-012-2134-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-012-2134-0