Abstract
The objective of this study was to assess the auditory performance of the neural structures in response to controlled electrical stimulation period. A prospective cohort study focused on the intracochlear electrical stimulation parameters and hearing performance of patients suffering different cochlear malformations who were treated by cochlear implants constituted the study design. The study sample constituted 16 patients, suffering profound prelingual hearing impairment, diagnosed on the basis of radiological criteria as having an inner ear malformation, and who underwent cochlear implantation and were followed for 24 months. Patients with common cavities, characterized by fewer nerve structures involved, less epithelial penetration, and deficient cochlear tonotopy distribution showed have higher thresholds and electrical charges than patients with cochlear hypoplasia, who in turn have higher thresholds than patients with minor malformations (p < 0.05). Furthermore, word perception was severely compromised in patients with a common cavity malformation and was also poor in patients with cochlear hypoplasia, who were unable to discriminate more than 50% of the words and relied on visual cues as a necessary aid to communication. Better results were reached by minor malformed inner ears. To conclude, the number of nerve structures involved, epithelial penetration and deficient cochlear tonotopy are responsible of inner ear functionality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cochlear implantation for inner ear malformations has been widely used in the last 15 years [1], and children with abnormal cochleovestibular anatomy are increasingly being considered as candidates. According to previous data [1, 2], cochlear malformations are expected to occur in 20% of children with sensorineural hearing impairment, with varying degrees and rates of progression of hearing loss. As a rule, the more severe the temporal bone deformity, the worse the impairment [2]. As first published by Jackler et al. [2] and confirmed by other authors [3, 4], inner ear malformations represent arrested cochlear development at particular stages of the embryological development, and are caused by genetic alterations, infections, exposure to ototoxic substances and radiation, among other factors. The developmental arrest is then manifested after the individual is born.
The embryological development of the inner ear is well known [5–7] and forms the basis of current classifications of congenital malformations [2–4]. Currently, Sennaroglu’s classification [3] seems to be the most accurate as it uses the most sophisticated imaging techniques, computed tomography (CT) and magnetic resonance imaging (MRI) to identify inner ear malformations and correlate the embryological stage with the malformation. Although embryological development, morphological data and the results and complications of cochlear implantation have been widely reported in the literature, some basic aspects of the distribution of neural structures and their auditory performance after electrical stimulation remain unknown.
The aim of this study was to assess the auditory performance of the neural structures in response to controlled electrical stimulation period.
Patients and methods
To determine the overall hearing performance, the cochlear neural specialization to sound and to electrical stimulation and cochlear tonotopy of different inner ear malformations, we designed a prospective, observational cohort study of 16 patients, suffering from different inner ear malformations, who underwent cochlear implantation and were followed-up for 24 months at the San Cecilio University Hospital in Granada, a tertiary reference center serving patients with cochlear implants in the autonomous community of Andalusia (southern Spain). All the 16 patients had profound prelingual hearing impairment and were diagnosed on the basis of radiologic criteria (CT and MRI) as having an inner ear malformation. We classified the malformations according to the system proposed by Sennaroglu and colleagues: (1) common cavity (three patients, 18.75%), (2) cochlear hypoplasia (two patients, 12.5%), (3) incomplete partition (four patients, 25%), and (4) minor vestibular malformations including dilatations (four patients, 25%) and duct malformations (three patients, 18.75%). For comparison, we studied 32 match-paired control patients. These control patients match each malformation patient on the age at deafness, the age at implantation, sex and even cochlear implant device. Table 1 summarizes the epidemiologic data in these two groups. There were no significant differences between the groups in age at cochlear implantation, sex or even hearing thresholds, although they differed in the prevalence of associated illnesses, which were more frequent in the group of patients with malformations. In this group, one patient each had one of the following diagnoses: congenital cytomegalovirus infection, congenital heart defect, hyperglycemia during pregnancy, perinatal infection, instrumental delivery, and maternal salmonellosis during pregnancy. All patients were implanted with Med-el devices (Combi 40+, Pulsar Ci100 and Sonata Ti100) and standard electrode arrays except those patients suffering common cavities and cochlear hypoplasias which were implanted with compressed electrode arrays.
All patients in both groups underwent pre-operative radiologic examination (CT and MRI) and hearing tests with click-evoked auditory brainstem responses. In order to asses the correct electrode insertion, the intra-operative assessment included Stenvers X-ray imaging, and cochlear implant objective measures such us the impedance telemetry, performed in all patients, and the compound action potentials electrically evoked (eCAP) which were only measured in those patients implanted with Med-el Pulsar Ci100 and Sonata Ti100 cochlear implants. After surgery, intracochlear electrical stimulation parameters (threshold, maximum comfort level, electrical pulse width, minimum electrical charge needed to evoke an auditory signal, and number of active electrodes) and auditory skills (listening progress profile and monosyllabic-trochee-polysyllabic word test, translated and validated for the use with Spanish speakers) were tested. Patients’ hearing skills were evaluated and the cochlear implant settings were adjusted frequently during 24 months.
Data for the two groups were compared with SPSS v.17 software. Because of the sample size, we used nonparametric statistical tests: Mann–Whitney U and Kruskal–Wallis test.
Results
Table 2 shows the mean hearing thresholds before cochlear implantation in each group and the 24 month-postoperative electrical stimulation values (threshold, minimum electric charge needed to evoke an auditory signal and number of active electrodes). Deactivated or inactive electrodes were not used to calculate mean values for the variables reported.
There were no statistically significant differences in mean hearing thresholds between patients with and without malformations. All patients had severe, profound hearing impairment (Table 1). Both groups differed significantly (Mann–Whitney U test, p < 0.05), however, in mean electrical threshold and minimum electrical charge at threshold 24 months after surgery. Although our analysis of mean hearing thresholds did not distinguish between different malformations, mean electric charge at threshold was higher in patients with more severe malformations (common cavity and cochlear hypoplasia) than less severe malformations (incomplete partition and vestibular malformations), and these differences approached significance (Mann–Whitney U test 0.05 < p < 0.1).
Although the cochlear implant insertion was complete in all patients, the mean number of active electrodes was significantly lower in patients with inner ear malformations (Mann–Whitney U test p < 0.05). In patients with a common cavity malformation the mean number of electrodes activated was lower than in patients with other malformations (Mann–Whitney U test p < 0.05).
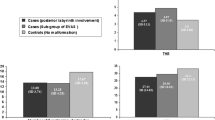
Hearing skills measured through LiP and MTP tests 24 months after cochlear implantation (Fig. 1) showed improvement after cochlear implantation. Also, mean scores after cochlear implantation were significantly lower in patients with an inner ear malformation than in patients with no malformation (Mann–Whitney U test p < 0.05). The only exceptions to this trend were the slightly but nonsignificantly higher LiP and MPT3 test scores 24 months after implantation in patients with incomplete partition and vestibular malformation. Patients with a common cavity malformation scored lower on all tests compared to control patients, whereas patients with cochlear hypoplasia scored lower on the MTP3 and MTP12 tests (Mann–Whitney U test p < 0.05). No statistical differences (Mann–Whitney U test p > 0.1) were found in MTP6 scores of patients suffering cochlear hypoplasia.
Discussion
The etiology, morphology and functional consequences of inner ear malformations have received renewed interest in the recent years with the advent of surgical treatment and rehabilitation with cochlear implants [1, 5–10]. Studies to date have offered new concepts regarding embryological development [5–7],which have been reflected in new morphological classifications whose effectiveness, limitations and complexities have been analyzed [2, 3]. The present study clarifies some basic aspects of the distribution and functioning of neural cochlear structures in malformed cochleas, based on histological analysis, on the responsiveness to intracochlear electrical stimulation and on speech discrimination tests.
Embryological development of the inner ear begins at 28–32 days with the appearance of nerve fibers that extend from the cochleovestibular ganglion to the otic epithelium. Thereafter, nerve fibers: (1) mature and increase in number, (2) penetrate toward the cochlear epithelium to give rise to ciliated cells, (3) continue to specialize, and, (4) distribute in an organized fashion in the modiolus of the cochlear turns to give rise to the final tonotopic array [2, 3, 5–7]. Functional maturity is attained with anatomical maturity of the inner ear [11].
The different inner ear malformations can appear as a result of the arrest in the embryological development at different stages. These arrests condition the presence or the absence of different structures in the mature inner ear. Even more, the maturity and also the functional characteristics of the inner ear structures may be then compromised. In this connection, our results show that patients with an inner ear malformation (all as a group and when considered according to the specific type of malformation) had significantly higher mean electrical thresholds than patients with no malformation. When we compared patients with specific types of malformation, we found that electrical thresholds were inversely related to the degree of cochlear differentiation, although the differences between types of malformation were not statistically significant. These results, which were unanticipated in part, were further corroborated by the electrical charge at threshold (Cu/s), a measurement that accurately quantifies the minimum energy needed for an electric pulse to evoke an auditory response. Patients with a common cavity malformation have higher threshold electrical charges than patients with cochlear hypoplasia, who in turn have higher thresholds than patients with minor malformations such as incomplete partition or vestibular malformations. The differences between these subgroups were statistically significant.
In patients with inner ear malformations, the higher electrical charges needed to evoke auditory responses and the limited battery voltage made it necessary to increase pulse duration, which resulted in a decreased stimulation rate. The decreased stimulation rate limited the hearing performance of patients with cochlear malformations receiving cochlear implants [12, 13]. In order to improve stimulation rates, stochastic behavior and auditory cell resynchronization, we deactivated those electrodes with higher charges [14].
Hearing skills paralleled electrical stimulation thresholds. Naturally, all patients in our study had significantly improved word perception and identification as a result of their cochlear implant and rehabilitation. It should be emphasized that the percentage gains in word perception and identification paralleled the degree of cochlear differentiation. These results are consistent, in part, with findings reported by other authors [8–10], although not all studies considered different malformations separately. The results were not distributed homogeneously across different inner ear malformations. Patients with vestibular malformations and minor malformations (incomplete partition or Mondini deformity) obtained scores similar to those of patients with hearing impairment but no malformation. In contrast, patients with major malformations (common cavity or cochlear hypoplasia) obtained significantly lower mean scores compared to other patients. Moreover, word perception was severely compromised in patients with a common cavity malformation (MTP3, MTP6 and MTP12), and was also poor in patients with cochlear hypoplasia, who were unable to discriminate more than 50% of the words and relied on visual cues as a necessary aid to communication. These findings are consistent with earlier results [10, 15, 16]. The reduced number of neuronal cells, their limited penetration in the epithelium, the lack of specialization and compromised tonotopy thus led to limited auditory performance even in response to electrical intracochlear stimulation.
Finally, although the severity degree of the malformation treated with cochlear implants seems to be the most important factor that affects hearing perception in cochlear malformation patients, many other variables may be relevant in these outcomes. These variables are related to: (1) the patient, such as the age at the onset of the hearing loss, the previous auditory experience, the age at implantation, co-morbidities and socioeconomical aspects, (2) the cochlear implant, such as the cochlear implant device, the electrical stimulation strategy, the electrode guide type, the number of inserted electrodes, the number of active electrodes and the electrode guide disposition inside the major cochlear malformations, and (3) the hearing rehabilitation. Most of these variable influences have been solved by matching controls to each malformation patient but fewer data was collected from the electrode guide insertion, the electrode guide disposition inside major malformations and the families’ socioeconomical characteristics. These variables may affect the present results and should be assessed in future studies.
References
Luntz M, Balkany T, Hodges AV, Telischi FF (1997) Cochlear implants in children with congenital inner ear malformations. Arch Otolaryngol Head Neck Surg 123:974–977
Jackler RK, Luxford WM, House WF (1987) Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope 97:2–14
Sennaroglu L, Saatci I (2002) A new classification for cochleovestibular malformations. Laryngoscope 112:2230–2241
Zheng Y, Schachern PA, Djalilian H, Paparella M (2002) Temporal bone histopathology related to cochlear implantation in congenital malformation of the bony cochlea. Otol Neurotol 23:181–186
Yasuda M, Yamada S, Uwabe C, Shiota K, Yasuda Y (2007) Three-dimensional analysis of inner ear development in human embryos. Anat Sci Int 82:156–163
Rinkwitz S, Bober E, Banker R (2001) Development of the vertebrate inner ear. Ann N Y Acad Sci 942:1–14
Barald KF, Kelley MW (2004) From placode to polarization: new tunes in inner ear development. Development 131:4119–4130
Asma A, Anouk H, Luc VH, Brokx JP, Cila U, Van De Heyning P (2010) Therapeutic approach in managing patients with large vestibular aqueduct syndrome (LVAS). Int J Pediatr Otorhinolaryngol 74:474–481
Chadha NK, James AL, Gordon KA, Blaser S, Papsin BC (2009) Bilateral cochlear implantation in children with anomalous cochleovestibular anatomy. Arch Otolaryngol Head Neck Surg 135(9):903–909
Kim LS, Jeong SW, Huh MJ, Park YD (2006) Cochlear implantation in children with inner ear malformations. Ann Otol Rhinol Laryngol 115:205–214
Herman P, Van Den Abbeele T, Portier F, Marianowski R, Copin H, Tran Ba Huy P (1997) Embryologie de l’oreille interne. In: EMC Oto-rhino-laryngologie. Paris: Elsevier Masson SAS 20-005-A-40
Loizou PC, Poroy O, Dorman M (2000) The effect of parametric variations of cochlear implant processors on speech understanding. Acoust Soc Am 108:790–802
Kiefer J, von Ilberg C, Rupprecht V, Hubner-Egner J, Knecht R (2000) Optimized speech understanding with the continuous interleaved sampling speech coding strategy in patients with cochlear implants: effect of variations in stimulation rate and number of channels. Ann Otol Rhinol Laryngol 109:1009–1020
Rubinstein JT, Hong R (2003) Signal coding in cochlear implants: exploiting stochastic effects of electrical stimulation. Ann Otol Rhinol Laryngol Suppl 191:14–19
Jackler RK, Luxford WM, House WF (1987) Sound detection with the cochlear implant in five ears of four children with congenital malformations of the cochlea. Laryngoscope 97:15–17
Blake C, Papsin MD (2005) Cochlear implantation in children with anomalous cochleovestibular anatomy. Laryngoscope. 115:1–19
Acknowledgments
The authors thank K. Shashok for translating the manuscript into English.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sainz, M., Garcia-Valdecasas, J., Fernandez, E. et al. Auditory maturity and hearing performance in inner ear malformations: a histological and electrical stimulation approach. Eur Arch Otorhinolaryngol 269, 1583–1587 (2012). https://doi.org/10.1007/s00405-011-1792-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-011-1792-7