Abstract
Ionizing radiation as a cancer therapy is associated with a variety of undesirable side effects. Consequently, radiotherapy can negatively affect neuromuscular function. Clinical observations have identified problems with swallowing and voice function. Our study aims to evaluate the impact of radiotherapy on laryngeal soft tissues using image analysis to quantify its effect on the structure of the vocalis and thyroarytenoid muscles. Case control study, retrospective analysis. We collected total laryngectomy specimens from six patients with persistent or recurrent cancer who had received preoperative radiotherapy (60–66 Gy). The control group consisted of total laryngectomy specimens from six patients who underwent surgery as primary treatment. Sampling of the specimens only included non-cancerous laryngeal tissue. Laryngeal histological slices were evaluated using digital morphometric analysis system. Percentage of fibrosis and density of muscle fibers within the thyroarytenoid muscle were evaluated in both groups. We found no significant quantitative differences in muscle fibrosis (7.92% vs. 7.52%, P > 0.1). Changes were rather qualitative and included changes in the organization of the muscular fibers. A significant reduction in muscle fibers, however, was observed in the samples from irradiated larynges (66.45% vs. 42.03%, P < 0.01). Our analysis suggests that radiotherapy is responsible for a significant reduction in muscle fibers in the thyroarytenoid muscle and that these changes occur during treatment or relatively early after its completion. Loss of muscle mass after irradiation correlates with clinical observations of muscle weakness and decreased function in patients who undergo radiotherapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer accounts for 3–5% of all malignancies, representing the sixth most frequent malignancy worldwide. The vast majority of these cancers are squamocellular histological variants [1]. Current management of head and neck cancer is multidisciplinary and includes some combination of surgery, radiotherapy, chemotherapy and/or biological therapy. Radiotherapy and surgery are considered the primary and most important treatment modalities. Despite its image as a less invasive treatment, ionizing radiation affects healthy as well as neoplastic tissues leading to numerous adverse effects [2–6]. Well-known side effects in the head and neck region include mucositis, xerostomia, osteoradionecrosis, trismus, reduction in taste perception, reduction in muscular strength, dysphagia and vocal weakness [7]. Indeed, our clinical experience, as well as that reported by others, suggests a wide variety and severity of swallowing disorders ranging from globus to morbid aspiration [8].
Several papers have addressed the effects of radiotherapy on swallowing function [9–11]. However, a literature search did not yield any publications dealing with post-radiotherapy laryngeal or pharyngeal changes at a tissue level. This prompted our research, which was aimed to ascertain the effects of radiation on non-cancerous laryngeal tissues by qualifying and quantifying changes in laryngeal intrinsic muscles, connective tissue and nerves using image analysis. We hypothesized that therapeutic ionizing radiation produces changes that include the loss of muscle fibers and an increase in collagen fibers, as well as architectural and morphologic changes in major nerves.
Materials and methods
Informed consent and approval by our institutional review board was obtained following established standards. Samples were obtained from patients undergoing a salvage laryngectomy as part of the treatment for laryngeal or pharyngeal cancer. We evaluated histological sections obtained from non-cancerous laryngeal tissues only. Our study cohort comprised patients who received preoperative radiotherapy (60–66 Gy) to the area of larynx. The time span between the completion of the radiotherapy and the laryngectomy was somewhat variable, ranging from 7–15 months. Of the selected six cohort patients, three had completed their radiation therapy 7 months, one patient 12 months and two patients 15 months prior to the laryngectomy. One patient was given concomitant chemoradiotherapy including cisplatin and 5-fluorouracil (intravenous cisplatin 100 mg/m2 days 1, 5 and fluorouracil 1,000 mg/m2 days 1–4, weeks 1 and 4). One patient was given concomitant cetuximab (intravenous 400 mg/m2 1 week before radiotherapy, then 250 mg/m2 weekly). Four patients were given radiotherapy alone. All patients received conventional (not intensity modulated) radiotherapy using cobalt and a linear accelerator dosed at 2 Gy per day administered 5 days a week.
A control cohort consisted of stage-matched patients who have not undergone radiotherapy to this area. All patients in the study were smokers or ex-smokers (Table 1).
Harvested tissue was fixed in 10% formaldehyde and embedded in paraffin. Transverse histological sections were obtained from the vocal folds (muscle and connective tissue), vocal process of the arytenoid, cricoarytenoid joint and superior and recurrent laryngeal nerves. We stained the histological slides with hematoxylin–eosin and Van Gieson’s stains.
Pictures were taken with a color CCD camera (Olympus ColorWiew III, Hamburg, Germany) attached to a microscope (Olympus IMT-4, Olympus Europa GmbH, Hamburg, Germany). A computerized morphometric program (Olympus AnalySIS, Olympus Europa GmbH, Hamburg, Germany) calibrated with a glass microscopic scale (Carl Zeiss, Jena, Germany) controlled the CCD camera. Background correction was used to remove dark corners and unwanted background (blurred microscope optics) from scanned images. A reference image for this procedure was taken in identical light conditions. All subsequent picture modifications and measurements were done using this system.
We used white balance and RGB adjustment, for precise image segmentation. Color tone was subsequently digitally modified for some images (muscle and fibrosis). Objects (muscle and fibrosis) were segmented by thresholding particular colors. The software (Olympus AnalySIS, Olympus Europa GmbH, Hamburg, Germany) automatically calculated the area of the defined (thresholded) objects and the entire observed area. Results were evaluated in Excel (Microsoft Corp, Seattle, Washington) calculating the mean, standard deviation and t-test. In cases where the objects could not be separated by thresholding (i.e., nerve evaluation), the objects were defined manually using the computer mouse. Evaluation of all other tissues followed the same procedures previously described for the imaging and analysis of the muscle tissue.
Muscle and fibrous tissues were evaluated using 50× magnification (Figs. 1, 2). An analysis of the muscle fibers and fibrous tissue, as well as their distribution within each area, was, respectively, determined using the computer software. Muscle tissue and fibrosis in both groups were evaluated at a “standard” transverse section of the middle third of the thyroarytenoid muscle the muscle (middle third of the vocal fold).
A transverse section of the superior and recurrent laryngeal nerves was evaluated using 100× magnification (Figs. 3, 4). Thickness of the perineurium was measured in the “standard” transverse sections. Average thickness of perineurium was measured manually at 15 points within regular intervals. Areas that included fibroblast nuclei were disproportionally thicker than other areas of the perineurium; therefore, their measurements were excluded from the database. Stated differently, we only measured those areas without nuclei to make the comparison homogeneous.
Results
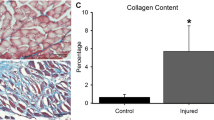
A significant reduction in muscle fibers was observed in the tissue of irradiated patients in comparison with the tissue of non-irradiated patients. Average area occupied by muscle fibers in the selected region was 66.45% in non-irradiated patients (Fig. 1) and 42.03% in patients after radiotherapy (Fig. 2). This difference was statistically significant P < 0.001.
We did not observe any statistically significant difference in the percentage of collagen fibers between irradiated and non-irradiated tissues. The average percentage of collagen fibers was 7.92% in non-irradiated patients (Fig. 1) and 7.52% in patients who received radiotherapy (Fig. 2). This difference was not statistically significant (P > 0.1). We did detect, however, qualitative changes including changes in the organization of fibrils and widening of the spaces among the muscle fibers.
We evaluated twelve recurrent laryngeal nerves and three superior laryngeal nerves obtained from irradiated patients and twelve recurrent nerves obtained from non-irradiated patients. The ratio of the thickness of the perineurium to the area of the nerve fibers (without perineurium) was also compared at the cross section. Average of the perineurial area-to-nerve area ratios was 0.047 in non-irradiated patients and 0.030 in patients after irradiation (Figs. 3, 4). This difference was statistically significant P < 0.05. It should be noted that differences in the diameter of the area of the nerves fibers could not be ascertained accurately with our software program; thus, we had to accept the ratio of perineurium to nerve fibers.
Discussion
Radiotherapy is one of the primary treatments for malignant head and neck neoplasms. Similar to other treatments, radiotherapy aims to cure while causing as few complications and side effects as possible. Effects of radiotherapy, and consequently its results, are dose dependent. To achieve its goal, it is necessary to target all the tumor cells with an intensity that leads to cell death or to their inability to replicate (through DNA breakage). Healthy surrounding tissues are spared as much as possible, thereby receiving the smallest possible dose of the radiation. Among other factors, the effectiveness of radiotherapy also depends on the ability of the diagnostic methods to accurately demonstrate the extent of disease, thereby avoiding geographical misses and sparing normal tissues.
Radiotherapy techniques have evolved since the mid-twentieth century when linear accelerators, betatrons and cobalt techniques were sequentially introduced. All these techniques provided a better dose distribution than conventional X-ray therapy. In the 1970s and 1980s, the introduction of CT-assisted treatment planning provided better tumor localization and improved the accuracy of the treatment fields. Currently, the standard is to use special spiral CTs (CT simulators) with planning software. Intensity modulated radiotherapy (IMRT) is a current technique that aims to reduce late toxicity while achieving locoregional control [12]. Another area of interest is the use of radioprotectors to increase in tolerance of healthy tissues. Nonetheless, even with the most advanced current techniques, irradiation of surrounding tissues carries a risk of acute and late complications. New schemes of fractionation have been developed to lower the dose absorbed by normal surrounding tissues; thus, the volume of irradiated tissues is now less than that of previous modalities.
Concomitant administration of chemotherapy increases the irradiation effectiveness, but also increases the frequency and severity of side effects. Cumulative mucosal toxicity is a significant problem and can lead to permanent dysphagia due to stenosis (i.e., mechanical obstruction) and other difficulties with the transfer of the bolus [6]. Dysphagia after chemoradiation treatment is complex and multifactorial and is also associated with reduced epiglottis inversion, delayed onset of swallowing, discoordinated propulsion, weak or incompetent laryngeal closure, incomplete opening of the upper esophageal sphincter, and inadequate retraction of the base of tongue that leads to reduced contact with the posterior pharyngeal wall (weak propulsion) [13]. In addition, the sensory function and afferent reflexes are significantly reduced. This combination leads to residue in the piriform sinuses and valecullae and a higher risk of prandial and postprandial [8].
Damage to healthy head and neck tissues resulting from radiotherapy is rarely addressed in the medical literature, although the general recommendation is not to exceed a radiation dose greater then 70 Gy [14]. Clinical impressions suggest that radiation-induced damage to nerves, motor and sensory, is underestimated and poorly understood. Low et al. monitored the function of vestibulocochlear nerve before, during and after radiotherapy for nasopharyngeal carcinoma using BSAER (brainstem auditory evoked responses). There was no change in latency of waves I-III-V intervals during or after radiotherapy on a group of 27 patients. He concluded that radiation produces no change in 8th cranial nerve function [15]. Conversely, Vujaskovič studied structural changes in peripheral nerves after intraoperative irradiation. Electron microscopic analysis showed an increase density of microtubules and accumulation of microfilaments but no changes in myelin. His study suggests that these changes were induced by radiation-induced hypoxia that subsequently lead to damage to the nerve fibers. These changes occurred when exposure exceeded 20 Gy [16]. This is one of the possible mechanisms leading to neuropathy and consequent swallowing disorders after radiotherapy. Our results showed a statistically significant reduction in the thickness of laryngeal nerve perineurium. However, the etiology of this change is unclear. Other possible explanations include a reduction in blood supply due to radiation-induced vascular damage, impaired blood supply due to endothelial fibrosis, interstitial swelling and direct damage to the nerve fibers. Our methods, histological staining and software program did not allow differentiation of these possible causes.
Others have addressed the effects of radiation therapy over muscle. Hsu et al. studied early and late effects of radiotherapy in muscle tissue using a rat model. Forty rats were irradiated using cobalt fractionation radiation therapy and subsequently a linear accelerator dose of 2 Gy to total of 80 Gy. Biopsies of the gastrocnemius muscle were taken at different intervals from the first day until 12 months after the completion of radiotherapy. Progressive structural changes were monitored using light and electron microscopy. Morphological changes began soon after completing the treatment. Bleeding, leukocyte infiltration and overt vascular destruction were observed. Throughout the reporting period, the investigators noted increase in collagen deposition. Signs of muscle regeneration still were observed 12 months after the completion of radiotherapy [17].
Powers et al. used histomorphometry to follow changes in the psoas muscle after external radiotherapy of 50 to 80 Gy in a canine model. They reported a decline in the percentage of muscle fibers and capillaries. Increase in connective tissue correlated with the dose of radiation. Muscle damage was characterized by a reduction in the number of muscle fibers, reduction in fibers thickness, bleeding into the tissues and coagulation necrosis and fibrosis [18].
In our observation, the decrease in muscle fibers after radiotherapy is associated with interstitial edema and mild focal atrophy of muscle fibers (different diameters of particular fibers and reduced overall diameter). Unlike others, we did not find macroscopic changes in the amount of collagen but observed changes in the organization of the fibrils (loss of their linear structure and organization).
We recognize several flaws in our study. Our patient population is small and heterogenous. In addition, the use of concomitant chemotherapy and biologicals is a confounding factor. Other considerations, such as malnutrition, prior damage to the microcirculation brought by smoking, high blood pressure or diabetes mellitus, may play a role. Further investigations and expansion of our study to include the results of multiple institutions and studying the possible differences in patients receiving IMRT are warranted. Similarly, improvements in software may allow the differentiation of changes in the nerve fibers. Nonetheless, our measurements complement the existing literature and may serve as another piece in the mosaic of knowledge that can lead to improved quality of life of the head and neck cancer patients.
Conclusion
Swallowing and voice disorders after irradiation seem to be caused, at least in part, by a reduction in muscular mass and by changes in the laryngeal nerves. Our data corroborated a reduction in the muscular mass of the thyroarytenoid muscle associated with interstitial edema and atrophy of muscle fibers. Ratio of perineurium to nerve fibers was higher in non-irradiated patients showing a relative reduction in perineurium width after irradiation. A multi-institutional study is warranted to analyze these findings with greater statistical power and to better control for other variables such as the use of IMRT, chemoradiotherapy and timing of the treatment.
References
Watkinson CJ, Gaze MN, Wilson JA (2000) Head and neck surgery. Butterworth-Heinemann, Oxford, UK
Higgins KM, Wang JR (2008) State of head and neck surgical oncology research–a review and critical appraisal of landmark studies. Head Neck 12:1636–1642
Schneider U, Lomax A, Pemler P et al (2006) The impact of IMRT and proton radiotherapy on secondary cancer incidence. Strahlenter Onkol 11:647–652
Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP (2003) Oral sequaleae of head and neck radiotherapy. Crit Rev Oral Biol Med 14:199–212
Mintz DR, Gullane PJ, Thomson DH, Ruby RR (1981) Perichondritis of the larynx following radiation. Otolaryngol Head Neck Surg 89:550–554
Cooper JS, Fu K, Marks J, Silverman S (1995) Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys 5:1141–1164
Platteaux N, Dirix P, Dejaeger E, Nuyts S (2009) Dysphagia in head and neck cancer patients treated with chemoradiotherapy. Dysphagia 2009, Aug 27. [Epub ahead of print]
Eisbruch A, Lyden T, Bradford CR et al (2002) Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 53:4–5
Simentaal AA, Carrau RL (2004) Assessment of swallowing function in patients with head and neck cancer. Curr Oncol Rep 6:162–165
Eisbruch A, Schwartz M, Rasch C et al (2004) Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 60:1425–1439
Nguyen NP, Moltz CC, Frank C et al (2004) Dysphagia following chemoradiation for locally advanced head and neck cancer. Ann Oncol 15:363–364
Dubinsky P (2007) Technologický pokrok v rádioterapii. Onkológia (Bratisl.) 6:342–344
Logemann JA (1998) Evaluation and treatment of swallowing disorders. Pro-ed, Austin, USA
Franzel W, Gerlach R (2009) The irradiation action on human dental tissue by X-rays and electrons—a nanoindenter study. Z Med Phys 19:5–10
Low WK, Burgess R, Fong KW, Wang DY (2005) Effect of radiotherapy on retro-cochlear auditory pathways. Laryngoscope 115:1823–1826
Vujaskovic Z (1997) Structural and physiological properties of peripheral nerves after intraoperative irradiation. J Peripher Nerv Syst 2:343–349
Hsu HY, Chay C-Y, Lee MS (1998) Radiation-induced muscle damage in rats after fractionated high-dose irradiation. Radiat Res 149:482–486
Powers BE, Gilette EL, Gilette SL, LeCourteur LA, Withrow SJ (1991) Muscle injury following experimental intraoperative irradiation. Int J Radiat Oncol Biol Phys 20:463–471
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tedla, M., Valach, M., Carrau, R.L. et al. Impact of radiotherapy on laryngeal intrinsic muscles. Eur Arch Otorhinolaryngol 269, 953–958 (2012). https://doi.org/10.1007/s00405-011-1686-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-011-1686-8