Abstract
The clinical presentation of low flow vascular malformations of the head and neck (LFVM) can range from a birthmark to severe disfigurement, functional impairment or relevant hemorrhage. The values of Brightness mode (B-mode) ultrasound and Doppler sonography in the investigation, identifying and differentiating of these lesions has been sparingly documented in the literature. This study evaluates the sonografic features of different morphological subtypes of LFVM. This is a 2-year retrospective study of 51 patients who presented with LFVM based on routine ultrasound exam in the context of their clinical consultation. Diagnosis was based on the clinical and histological findings. B-mode, color coded duplex and spectral Doppler measurements were performed for venous, lymphatic, capillary, and mixed venous-lymphatic lesions of the head and neck. The echogenicity of the majority of venous malformations was heterogenic, of most lymphatic malformations hypoechoic, and of all capillary malformations isoechoic. Blood flow was detected in only 11 cases (36.7%) of venous malformations with a monophasic pattern. There was a statistical significant difference in the mean minimum and maximum Doppler shifts between venous and lymphatic malformation for cases when the blood flow was evident. No statistical significant difference in Doppler parameters existed between capillary and lymphatic, neither between venous and capillary nor mixed malformations. Phleboliths were present in eight cases (26.7%) of venous malformations and were not detectable in any other subtype of LFVM. The detection of flow in ultrasound was only possible in a small portion of LFVM. When considering differentiating among LFVM, features such as the echogenecity, spectral Doppler wave forms, and the evidence of phleboliths contribute to establish the correct diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vascular anomalies have generally been classified by the international society for the study of vascular anomalies (ISSVA) into vascular tumors and vascular malformations on the basis of their natural biologic behavior and functional hemodynamic peculiarities [1]. Unlike vascular tumors (hemangiomas), vascular malformations do not undergo spontaneous involution with age and their endothelia do not undergo hyper-proliferation [1–3]. They remain stable but factors such as trauma or hormonal imbalance may stimulate their expansion which is usually slow. An updated classification of the vascular malformations for therapeutic and prognostic purposes has been proposed [2, 3]. Beside morphological differentiation, vascular malformations are sub-classified into high-flow (arteriovenous fistula, arteriovenous malformation) and low-flow (venous, capillary, lymphatic and combined forms) malformations due to their hemodynamic properties, which are of utmost relevance to clinical practice [1–4].
This study focuses on sonographic evaluation of low-flow vascular malformations of the head and neck (LFVM). Both minimally invasive and non invasive tools have been used to investigate LFVM. Contrast enhancement computerized tomographic (CT) scan, Magnetic resonance imaging (MRI) and ultrasound scan are the non-invasive imaging tools which have been commonly used in the evaluation of these patients. Ultrasound is easily available, economical, and rapid mode of investigation. Therefore, it is definitely of great clinical importance to learn more about the sonographic features of LFVM. However, the values of Brightness mode (B-mode) and Doppler ultrasound in the investigation, identifying and differentiating especially of LFVM has been sparingly documented in the literature. This study, therefore, evaluates the value of B-mode sonography in the diagnosis and management of low-flow vascular malformations of the head and neck region relating to its morphological subtypes and also analyses their properties in Doppler ultrasound.
Patients and methods
This is a 2-year retrospective study from August 2008 to July 2010 of patients who presented with LFVM and were examined with ultrasound in the context of their clinical consultation. The study was carried out at the Angioma Center, Department of Otolaryngology, Head and Neck Surgery, University of Marburg, Germany.
The lesions were categorized into venous-, lymphatic-, capillary-, and mixed (combined venous-capillary and combined venous-lymphatic) malformation based on their clinical presentation and histology in cases treated by conventional surgery. The information on patients’ biodata and clinical information were obtained as a part of routine documentation in patients’ medical record. This included presence of symptoms such as pain or bleeding, and location of the lesion in the head and neck region. Only lesions without previous interventions and measuring at least 2 cm in one dimension were included in the study. Ultrasound exam of all the patients was performed with Siemens Sonoline G60S™ with a frequency of 10.5 MHz (B-mode and color coded duplex) and Compumedics DWL2000™ Multi-Dop® Pro Doppler ultrasound with a frequency linear array transducer (8 MHz; CW mode; spectral Doppler). The transducer power output was set below 100 mW/cm2. The Doppler gain was at 50%, pulse repetition frequency at 4 kHz, without a wall filter. If more than one lesion was present in a patient, either the one which was greater in size or the lesion which was more superficial was included for the study. Patients with deep or intraosseous lesions which could not be delineated or properly detected by B-mode were excluded.
The histology reports of all patients who had surgical treatment of the lesions were also obtained. The ultrasound exams were performed in all these cases prior to treatment. The echogenicity was determined as the relation of at least three-quarters of the total volume of the lesion to the surrounding normal soft tissue and was classified as hypoechoic, isoechoic, hyperechoic, and heterogenous if it contained both hypoechoic and hyperechoic components. Further, the presence of phleboliths was recorded.
Color coded duplex sonography was performed in all cases to detect blood flow in each lesion. In addition, spectral Doppler tracing was taken at five different points of the lesions, if flow was detected in the duplex mode. The points for spectral Doppler measurements were standardized according to the following scheme: a total of five measurement points were defined including the center, the cranial, caudal, lateral, and medial border of each lesion. The minimum and maximum Doppler shift values, resistive (RI) and pulsatility (PI) indices were generated by the machine. The flow was recorded for a period of at least 5 s for each measurement point. The results were statistically analyzed using statistical package for social sciences (SPSS) version 16. The mean values from the spectral Doppler parameters taken at the five different points were used for the calculation. Chi-square test was used to study the association between the clinical diagnosis and the sonographic features using the mean values of minimum and maximum Doppler shift, the mean resistive and pulsatility indices. A two-sided Fisher exact test was used to study if the spectral Doppler findings differ from one sub-type of LFVM lesion to the other. The level of statistical significance was determined at p < 0.05 and confidence interval at 95%.
Results
Fifty-one patients were studied, this included 28 (54.9%) male and 23 (45.1%) female with a sex ratio of 1.22: 1 (M:F). Their age ranged from 0.5 to 71 years with a mean age of 26.3 years SD ± 19.3. Nine (17.7%) patients presented with pain while three (5.9%) patients with a history of associated bleeding from the lesion. The anatomical location of these lesions in the head and neck is presented in Table 1. Figure 1 displays the number of different subtypes of LFVM.
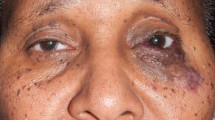
The morphologic appearances of the subtypes of LFVM in B-mode ultrasound are presented in Table 2. The majority of venous malformations (21 of 30 cases) showed a mixed echogenicity and was referred to as heterogenic (Fig. 2a), whereas the largest proportion of the lymphatic was hypoechoic. The echo texture of all capillary malformations resembled that of the surrounding subcutaneous tissue, hence isoechoic. Phleboliths were present in eight cases of venous malformations (26.7%). They were not detectable in any other subtype of LFVM.
Thirty-six (70.6%) had one form of therapeutic intervention, while the remaining 17 cases were observed. Nd:YAG laser therapy or sclerotherapy with bleomycin was performed in 21 (70%) of venous malformation cases and 2 (66.7%) of the mixed. Surgical excision of the lesion was performed in 13 cases and tissues were obtained for histological examination of which 8 (72.7%) were lymphatic malformations, 2 (18.2%) were venous, 2 (18.2%) were capillary, and 1 (9.1%) was mixed venous-lymphatic. There was no discrepancy between the clinical diagnoses and the results of the histological examination. Color coded duplex revealed a flow in 11 (36.7%) cases of venous malformations, two of capillary (28.6%), and two (18.2%) cases of lymphatic malformations. The Doppler spectrum was monophasic in venous (Fig. 3), lymphatic (Fig. 4), and mixed malformations and was biphasic in the two cases of capillary malformations (Fig. 5). The mean value of maximum Doppler shift in venous malformation ranged from 0.31 to 1.23 kHz (mean 1.21 kHz), with a mean RI of 0.87, mean PI of 2.5. The maximum Doppler shift of lymphatic malformations ranged from 0 to 0.2 kHz (mean 0.1 kHz), with a mean RI of 0.99 and mean PI of 1.85 (Table 3). The patients with lymphatic malformation have shown mean PI lower than and mean RI greater than those of other subtypes of low flow vascular malformations without statistical significance (p = 0.071, p = 0.078). There was a statistical significant difference in the mean minimum and maximum Doppler shifts between venous malformation and lymphatic malformation only (p = 0.041 and p = 0.032, respectively). However, no statistical significant difference in spectral Doppler parameters existed between other sub-types LFVM (p = 0.106 between capillary malformations and lymphatic malformations, p = 0.172 between venous malformation and capillary malformation).
Discussion
Low-flow vascular malformations of the head and neck (LFVM) in general are relatively rare lesions. Although the clinical diagnosis may be challenging in a clinical practice setting where such patients are not regularly managed, there is, however, no such difficulty in a specialized referrer centre noted for management of vascular anomalies. Management of LFVM often consists in sclerosing therapy, laser therapy or clinical observation. Therefore, it is not always justifiable to seek a biopsy. Because of this fact, we have explicitly decided to also include cases without histology in the present study. This is further supported by the concordance of the clinical and the histological diagnosis of the lesions in this study, which had a surgical treatment.
Venous malformations have been reported to be the commonest LFVM [5] and this has been confirmed by this study in which about 60% of the cases are venous malformation. These lesions do not proliferate and neither do regress spontaneously [5–7]. They are formed from tortuous, communicating venules, and larger venous vessels (Fig. 2b). The overlying skin or mucosa may be bluish stained and is usually without warmth, pulsation or audible bruit [7]. These can communicate directly with normal venous system; sometimes they present with complex lesions which can permeate any organ system through interconnected channels. Venous malformations may show an impressive expansion during valsalva maneuvers which is an important differentiating property from other non-venous LFVM [7].
Capillary malformations are the most common cutaneous vascular malformation appearing as red or purple macular lesions [8, 9]. They occur commonly on the face and become increasingly nodular with age. They can exhibit a segmental distribution pattern mimicking dermatomes, and may also be associated with developmental defects such as Cobb syndrome, encephalocele, Sturge–Weber–Klippel–Trenaunay syndrome and the so-called hyperkeratotic cutaneous capillary-venous malformation. Even though the pathogenesis is not well understood, it has been mapped to chromosome 5q14-21, showing a defect in the RASA1 gene [9]. However, Breugem and coworkers [10] have suggested that the pathologic abnormalities of capillary malformations appear to be located in postcapillary venules rather than the capillaries themselves.
Lymphatic malformation is a congenital, non-neoplastic lesion which commonly manifests at birth in the head and neck region. The sub-classification of lymphatic malformation into macrocystic and microcystic has been favored because of its prognostic and therapeutic importance [11]. In the head and neck region, its development has been proposed to either be from endothelial out-buddings from the jugular sac which spread centrifugally to form the lymphatic system or from mesenchymal clefts in the venous plexus reticulum that spread centripetally toward the jugular sac [12]. They may or may not be associated with the lymphatic drainage problem and more than two-third of lymphatic malformations are found in the soft tissue of the neck [1]. Unlike venous malformations, they do not expand during valsalva maneuvers, but are often associated with inflammations and internal hemorrhage. A large group of LFVM could also present in a combined form consisting of venous, capillary, and lymphatic components. A possible explanation for the occurrence of these combined forms is either their close relationship in their developmental history or the generation of potential short circuits by various factors, such as, e.g., iatrogenic or after inflammatory processes.
A detailed evaluation is essential for correct diagnosis, optimized treatment planning, and outcome for these lesions. Treatment is required in instances such as disfigurement, pain due to infection, thrombus formation within the lesion or irritation of nearby nerves, pressure on articular capsules, localized intravascular coagulopathy with bleeding and whenever functional impairment occurs [13].
Ultrasound is a very useful tool in demonstrating vascularization and vascular flow in vascular anomalies [14–19]. Doppler investigations of the vascular system are nowadays simple routine procedures in various medical disciplines such as neurology and angiology. It has a cost advantage, enjoys easy accessibility and also avoids needle prick pain and possible reactions to contrast materials which may be experienced in contrast enhanced CT or MRI. The distinction between arterial and venous flow is not challenging in clinical practice, but the ultrasound diagnostic properties of LFVM and differences between the subtypes of LFVM are still largely unknown and rarely described. Doppler ultrasound detects the flow velocity of the corpuscular elements in the blood. B-mode ultrasound, color coded, and spectral Doppler imaging together are able to demonstrate the arrangement pattern, the flow irregularities (stasis or acceleration) and flow velocity within vascular lesions [14], and these properties should be investigated at this point.
The variation of the imaging appearance of LFVM on B-mode depends largely on the relative proportion of the vessel wall components and its luminal components. When the luminal components predominate, there may appear as multiple cystic or isolated dilated spaces. The B-mode ultrasound appearance of venous malformations is a spongy-like lesion with variable separating walls due to the tortuous nature of the containing variable sized vessels. This gives it the heterogenous appearance as was seen in 70% of cases of venous malformation in this study. Trop and coworkers [20] have reported on 80% cases of venous malformation showing a heterogenous appearance. In contrast, B-mode morphologic appearance of the majority of lymphatic malformations was homogenous and hypoechoic and all capillary malformations were homogenous and isoechoic.
It has been reported that LFVM typically show no flow signals at rest [21]. However, flow was present in 11 (36.7%) cases of venous malformation and all showed a monophasic pattern on spectral Doppler (Fig. 3). The majority of the venous malformation did not show any evidence of flow signals at rest and on compression. The absence of flow in these cases may be due to limitations of the ultrasound machine or factors such as stasis of blood, presence of thrombosis, phleboliths or calcification within the lesion. This is considered as a possibility as there was no demonstration of flow signal in eight patients with presence of phleboliths in this study.
Trop and coworkers [20] have detected biphasic low velocity blood flow spectra in 6% of examined venous malformation by Doppler ultrasound and explain this observation with the presence of capillary components in mixed capillary-venous malformations. Indeed, in contrast to venous and lymphatic malformations, the Doppler spectrum in both “flow positive” cases of capillary malformations was biphasic (Fig. 5). They further have calculated an average flow velocity of 0.22 kHz on 43 venous malformations. The mean value for maximum Doppler shift in venous malformation in our study ranged from 0.31 to 1.23 kHz. This discrepancy can be explained by the different methodology (the measurements were carried out on five fixed, defined points of each lesion) and machine settings than the aforementioned authors.
Surprisingly, flow signal was detected in two cases with lymphatic malformations. The presence of venous vessels on the wall of the lymphatic cysts, as was evident during the resection of a lymphatic malformation of the parotid gland (Fig. 6a, b showing ectatic venous vessel attached to the cystic wall of the lymphatic malformation of the parotid gland) may explain this observation. Even though the cysts of lymphatic malformation are not significantly perfused, our group has reported how venous or arterial vessels may be located in immediate neighborhood to the cystic walls of lymphatic malformations [22]. It has been suggested that in an instance of demonstrable venous vessels on the cystic walls, veno-lymphatic malformation should be suspected [23, 24]. The small number of mixed malformations which were analyzed in this study does not justify taking a meaningful statement about their detailed properties on ultrasound. Further, the sole evidence of monophasic flow does not justify a diagnosis of mixed, e.g., venous-lymphatic malformation. The presence of vasa vasorum and, in particular, the close proximity of lymphatic to venous vessels is a much more acceptable assumption for this observation.
The evidence of phleboliths is pathognomic for venous malformations [5, 17], but they were present in only eight cases. Therefore, one cannot rely on the detection of phleboliths as the only distinguishing feature of venous malformations. We had asked whether using the Doppler indices would provide a more detailed differentiation and whether Doppler parameters could be used as reference values for subtypes of LFVM. There was a statistical significant difference in the minimum and maximum Doppler shifts between venous malformation and lymphatic malformation. This fact is in concordance with the clinical features of these lesions. Other Doppler indices such as the pulsatility (PI) and resistive index (RI) have proven to be of value in certain clinical fields such as obstetrics or neurology. The patients with lymphatic malformation have shown mean PI lower than and mean RI greater than those of other subtypes of low flow vascular malformations, but we could not prove it statistically. The implication of above results is that taking into account the Doppler indices alone will not be sufficient for distinguishing among the LFVM. However, when in doubt between venous and lymphatic malformation, these parameters could be a useful distinguishing investigative tool. The diagnosis of vascular anomalies in the head and neck region is based primarily on the basis of clinical findings. When considering differentiating among LFVM, features such as the echogenecity, spectral Doppler wave forms, and the evidence of phleboliths contribute to establish the correct diagnosis.
References
Werner JA, Eivazi B, Folz BJ, Dünne AA (2006) State of the art of classification, diagnostics and therapy for cervicofacial hemangiomas and vascular malformations. Laryngorhinootologie 85:883–891
Eivazi B, Ardelean M, Bäumler W, Berlien HP, Cremer H, Elluru R, Koltai P, Olofsson J, Richter G, Schick B, Werner JA (2009) Update on hemangiomas and vascular malformations of the head and neck. Eur Arch Otorhinolaryngol 266:187–197
Mulliken JB, Glowacki J (1982) Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 69:412–422
Meyer JS, Hoffer FA, Barnes PD, Mulliken JB (1991) Biological classification of soft-tissue vascular anomalies: MR correlation. AJR 157:559–564
Eivazi B, Wiegand S, Pfützner W, Neff A, Kureck I, Roessler M, Werner JA (2009) Differential diagnosis of vascular malformations of the upper aerodigestive tract. Laryngorhinootologie 88:700–708
Abernethy LJ (2003) Classification and imaging of vascular malformations in children. Eur Radiol 13:2483–2497
Puig S, Caasati B, Staudenherz A, Paya K (2005) Vascular low-flow malformations in children: current concepts for classification, diagnosis and therapy. Eur J Radiol 53:35–45
Happle R (2008) What is a capillary malformation? J Am Acad Dermatol 59:1077–1079
Mulliken JB (1990) Cutaneous vascular anomalies. In: McCarthy JG (ed) Plastic surgery. WB Saunders, Philadelphia, pp 3191–3274
Breugem CC, Hennekam RC, van Gemert MJ, van der Horst CM (2005) Are capillary malformations neurovenular or purely neural? Plast Reconstr Surg 115:578–587
Wiegand S, Eivazi B, Zimmermann AP, Sesterhenn AM, Werner JA (2010) Sclerotherapy of lymphangiomas of the head and neck. Head Neck (in print)
Wiegand S, Eivazi B, Barth PJ, von Rautenfeld DB, Folz BJ, Mandic R, Werner JA (2008) Pathogenesis of lymphangiomas. Virchows Arch 453:1–8
Eivazi B, Wiegand S, Teymoortash A, Neff A, Werner JA (2010) Laser treatment of mucosal venous malformations of the upper aerodigestive tract in 50 patients. Lasers Med Sci 25:571–576
Yakes WF (2008) Diagnosis and man-agement of low-flow veno-lymphatic vas-cular malformations. Ces Radiol 62:131–145
Rózylo-Kalinowska I, Brodzisz A, Gałkowska E, Rózylo TK, Wieczorek AP (2002) Application of Doppler ultrasonography in congenital vascular lesions of the head and neck. Dentomaxillofac Radiol 31:2–6
Ahuja AT, Richards P, Wong KT, Yuen EH, King AD (2003) Accuracy of high-resolution sonography compared with magnetic resonance imaging in the diagnosis of head and neck venous vascular malformations. Clin Radiol 58:869–875
Yang WT, Ahuja A, Metreweli C (1997) Sonographic features of head and neck hemangiomas and vascular malformations: review of 23 patients. J Ultrasound Med 16:39–44
Dubois J, Garel L, David M, Powell J (2002) Vascular soft-tissue tumors in infancy: distinguishing features on Doppler sonography. Am J Roentgenol 178:1541–1545
Flis CM, Connor SE (2005) Imaging of head and neck venous malformations. Eur Radiol 15:2185–2193
Trop I, Dubois J, Guibaud L, Grignon A, Patriquin H, McCuaig C, Garel LA (1999) Soft tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology 212:841–845
Paltiel HJ, Burrows PE, Kozakewich HP, Zurakowski D, Mulliken JB (2000) Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology 214:747–754
Eivazi B, Teymoortash A, Wiegand S, Roessler M, Mandic R, Bien S, Werner JA (2010) Intralesional endoscopy of advanced lymphatic malformations of the head and neck: a new diagnostic approach and a potential therapeutic tool. Arch Otolaryngol Head Neck Surg 136:790–795
Harsha WJ, Crawford JV, Sorensen DM (2008) An unusual case of adult airway obstruction from a lymphovenous malformation. Ear Nose Throat J 87:402–404
Jackson IT, Carreno R, Potparic Z, Hussain K (1993) Hemangiomas, vascular malformations, and lymphovenous malformations: Classification and methods of treatment. Plast Reconstr Surg 91:1216–1230
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eivazi, B., Fasunla, A.J., Hundt, W. et al. Low flow vascular malformations of the head and neck: a study on brightness mode, color coded duplex and spectral Doppler sonography. Eur Arch Otorhinolaryngol 268, 1505–1511 (2011). https://doi.org/10.1007/s00405-011-1514-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-011-1514-1