Abstract
Eosinophil cationic protein (ECP), a potent cytotoxic molecule, is released by activated eosinophils. ECP has been suggested to be involved in tissue remodeling of allergic diseases. The ECP (RNase3) gene is a candidate gene in atopic diseases. RNase3 polymorphisms have been reported to have an association with atopy. We determined whether polymorphisms in the RNase3 gene are associated with allergic rhinitis in a Korean population. The Taqman assay, restriction fragment length polymorphism (PCR-RFLP), and high-resolution melt (HRM) were used for genotyping. Three single nucleotide polymorphisms (SNPs; g.−550A>G, g.371G>C, and g.499G>C) were identified. The genotype of the SNPs was analyzed in patients with allergic rhinitis and controls without allergic rhinitis. The genotype and allele frequencies were compared between both groups. The genotype frequencies of the g.−550A>G and g.371G>C SNPs were not significantly different between patients with allergic rhinitis and controls (P > 0.05). However, in patients with allergic rhinitis, the genotype and allele frequencies of the g.499G>C SNP of RNase 3 were significantly different from those of the control group (P < 001, P = 0.034, respectively). Haplotype analysis demonstrated the presence of the following five different (−550)-(+371)-(+499) major haplotypes: A-G-G, G-C-C, G-G-G, G-C-G, and A-G-C. The G-C-G haplotype was positively associated with allergic rhinitis (P = 0.048), while the G-G-G haplotype was negatively associated with allergic rhinitis (P = 0.004). Our study suggests that RNase3 polymorphisms are potentially associated with susceptibility to allergic rhinitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eosinophilic airway inflammation is a characteristic feature of allergy, and serum eosinophil cationic protein (s-ECP), a granular protein derived from activated eosinophils, reflects the degree of activation of the circulating eosinophilic pool in the body [8]. ECP is a truly multifunctional protein with cytotoxic and non-cytotoxic capabilities. ECP is a member of the eosinophil-associated RNase family [10, 11]. The ECP (RNase3) gene is located on human chromosome 14q11.2 [9]. We hypothesized that genetic polymorphisms in the human ECP gene could influence the development of allergic rhinitis. In order to test the hypothesis, we investigated three polymorphisms in the ECP gene and conducted an association study in patients with allergic rhinitis.
Materials and methods
Patients and DNA samples
On the basis of approval and informed consent from the Review Board of the School of Medicine of Wonkwang University, blood samples were obtained from 478 healthy controls (301 males and 177 females) and 440 patients with allergic rhinitis (275 males and 165 females). The mean ages of the controls and patients were 33.5 and 29.8 years, respectively. Genomic DNA was extracted from leukocytes in peripheral blood by a standard phenol–chloroform method or with a Genomic DNA Extraction kit (iNtRON Biotechnology, Seongnam, Korea), according to the manufacturer’s directions. The patients with allergic rhinitis were recruited from the outpatient clinic of Wonkwang University Hospital. The diagnosis was based on symptoms of sneezing, watery rhinorrhea, nasal obstruction, and the results of a positive skin test. The skin test was performed with six common aeroallergens from house dust mites, house dust, grass mix, tree pollens, animal dander, and molds (Torii, Tokyo, Japan). All of the patients with allergic rhinitis had a history of the symptoms and had a positive skin test. We carefully selected healthy control subjects. The controls were recruited from the general population who took a comprehensive medical examination at Wonkwang University Hospital. A detailed questionnaire was used to select subjects who had no evidence of any personal or family history of allergies. All of the control subjects were negative for allergic symptoms and had negative results on allergy testing. All subjects in this study were Korean and were living in the same geographic area.
TaqMan analysis
The assay reagents for the genomic (g.) −550A>G (rs2284954) single nucleotide polymorphism (SNP) of the RNase3 gene were designed by Applied Biosystems (Foster City, CA, USA). The reagents consisted of a 40× mix of unlabeled polymerase chain reaction (PCR) primer and TaqMan MGB probes (FAM and VIC dye-labeled; Table 1). The reaction condition was optimized in a 10 μl total volume with 0.125 μl 40× reagents, 5 μl 2× TaqMan Genotyping Master mix (Applied Biosystems), and 2 μl (50 ng) of genomic DNA. PCR conditions were as follows: 1 cycle at 95°C for 15 min; 50 cycles at 95°C for 10 s, and 60°C for 45 s. The PCR was performed in a Rotor-Gene thermal cycler RG6000 (Corbett Research, Sydney, Australia). The samples were read and analyzed using Rotor-Gene 1.7.40 software (Corbett Research).
Polymerase chain reaction-restriction fragment length polymorphism
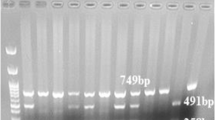
The RNase3 promoter, exon 1, and intron 1 regions containing the g.371G>C (rs2073342) polymorphic sites was partially amplified using the primer set (Table 1). An initial PCR denaturation step was performed at 95°C for 5 min, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at the melting temperature of each primer pair for 15 s, and extension at 72°C for 1 min, with a final 10 min extension at 72°C. The PCR products was digested with 4 U of PstI (NEB, Ipswich, MA, USA) for 12 h at 37°C, and then separated on 1.5% agarose gel and visualized under UV with ethidium bromide. When the GG genotype sequence was present, the PCR products (1,249 bp) took the form of two fragments (1,050 and 199 bp) by restriction enzyme digestion.
High-resolution melting analysis
The SNPs information of the RNase3 gene was derived from the NCBI SNP database. Genotype analysis was performed by high-resolution melting (HRM) analysis for the g.499C>G (rs2233860) SNP (Table 1). The 10 μl reaction mixture was made up using 2 μl of genomic DNA (50 ng), 1 μl of primer mix (containing 5 pmol of the forward and reverse primers), 0.25 μl of EvaGreen solution (Biotium, Hayward, CA, USA), and 5 μl of QuantiTect Probe PCR Kit (Qiagen, Valencia, CA, USA). PCR cycling and HRM analysis was performed on GeneTM 6000 (Corbett Research). The PCR cycling conditions were as follows: 1 cycle at 95°C for 15 min; 45 cycles at 95°C for 15 s; annealing conditions at 55°C for 20 s; and 72°C for 30 s. After completion of 45 cycles, melting curve data were generated by increasing the temperature from 77 to 95°C at 0.1°C/s and recording fluorescence. HRM curve analysis was performed using Rotor-Gene 1.7.40 software and the HRM algorithm that was provided.
Statistical analysis
The χ2 tests were applied to estimate the Hardy–Weinberg equilibrium (HWE). A pair-wise comparison of bi-allelic loci was employed for the analysis of linkage disequilibrium (LD). The haplotype frequencies of RNase3 for multiple loci were estimated using the expectation maximization (EM) algorithm with SNPAlyze software (DYNACOM, Yokohama, Japan). Logistic regression analyses were adapted to calculate odds ratios (95% confidence interval). A P value <0.05 was considered to indicate statistical significance.
Results
We identified three SNPs (g.−550A>G [rs2284954], g.371G>C [rs2073342], and g.499G>C [rs2233860]). We analyzed the genotype of these SNPs in patients with allergic rhinitis and controls without allergic rhinitis. The genotype and allele frequencies were compared between both groups (Table 2). The genotype frequencies of the g.−550A>G and g.371G>C SNPs were not significantly different between patients with allergic rhinitis and controls (P > 0.05). However, in patients with allergic rhinitis, the genotype frequencies of the g.499G>C SNP of RNase3 were significantly different from the control group (P < 001). The allele frequencies of the g.499G>C SNP were also significantly different from the control group (P = 0.034).
We also estimated the haplotype frequencies of the g.−550A>G, g.371G>C, and g.499G>C SNPs of RNase3 between controls and patients with allergic rhinitis. The haplotype analysis of the following 5 different (−550)-(+371)-(+499) major haplotypes (frequency > 0.05) are presented in Table 3: A-G-G, G-C-C, G-G-G, G-C-G, and A-G-C. The G-C-G haplotype was positively associated with allergic rhinitis (P = 0.048), while the G-G-G haplotype was negatively associated with allergic rhinitis (P = 0.004).
Discussion
The eosinophil granulocyte is an inflammatory cell which is thought to be important in parasite defense and wound healing [2]. The pathophysiologic role of the eosinophil has mostly been related to its presence and activation in allergic diseases, such as asthma and allergic rhinitis [1]. The activity of the eosinophil is to a large extent determined by the secretion of the basic proteins, ECP, eosinophil peroxidase (EPO), eosinophil protein X (EPX), and major basic protein (MBP) [7]. The biological activities of these proteins are many, but the cytotoxic activity of the molecules and their capacity to kill almost any cell, mammalian or non-mammalian, is of particular interest. The ECP seems to be one of the most potent of the basic proteins.
It is apparent that no single genetic risk factor is responsible for the development of allergic rhinitis. However, cumulative information in the field of genetics suggests that the development of a disease in an individual will depend upon the interaction of a number of genes of mild-moderate effect together with various environmental factors. The ECP gene may be a candidate gene in the development of allergies.
A Swedish study reported an association between the 434G allele (labeled g.371G>C in the present study) and allergy [2]. More recently, another study by the same group demonstrated an association between the 562G allele (labeled g.499G>C in the present study) and high cellular ECP content [3]. They reported that the association between the 371G and 499G alleles with allergy and cellular content of ECP suggests that the two alleles are involved in asthmatic/allergic inflammation. More recently, a Norway study analyzed three SNPs (−38C>A, 371G>C, and 499G>C) in RNase3 [8]. Despite similar allele frequencies, the Norwegian study could not reproduce the results of the Swedish association study, detecting an association between the 371G allele and allergy, but not asthma, although the 371G allele was associated with protection against the non-allergic asthma phenotype in the study [8]. The Norwegian study reported that the 499G allele is present in both the asthma/allergy-associated A-G-G haplotype of the −38C>A, 371G>C and 499G>C SNPs in RNase3 and the protective C-G-G haplotype detected in the study, raising the question of a possible haplotype effect rather than a SNP effect [8].
Although the genetic association studies were not directly comparable, the results of our study were different from previous studies [2, 3, 8]. The Swedish study showed that allergic subjects had a significantly higher prevalence of the homozygous 434G (labeled g.371G>C in the present study) genotype of the ECP gene and no subjects with reported allergic symptoms had the homozygous 434C genotype [2]; however, this study could not show the same results of the Swedish association study. Our study presents an association between the g.499G>C SNP and allergic rhinitis. In our study, both the 499GG and GC were not significantly different between patients with allergic rhinitis and controls (P > 0.05), but the 499CC was associated with allergic rhinitis (P < 0.001). This result means only the CC combination of the g.499G>C SNP may be related to the allergic rhinitis. The prevalence of allergic rhinitis was not more common among those with the 499GG and GC genotypes, whereas the 499CC genotype may be predictive for allergic rhinitis.
Based on our haplotype analysis, the present study demonstrates an association between the G-C-G haplotype (g.−551A>G, g.371C>G, and g.499G>C) and allergic rhinitis, whereas the haplotype that differs only in the g.371CG site (G-G-G haplotype) was associated with a reduced occurrence of allergic rhinitis, suggesting that the development of allergic rhinitis could be influenced by the SNP, as well as haplotype effects. Our findings show the CC combination of the g.499G>C SNP and/or G-C-G haplotype (g.−551A>G, g.371G>C, and g.499G>C) could be associated with susceptibility to allergic rhinitis. Thus, the functions of ECP could be operative in the development of allergic symptoms and affected by the amino acid change. In order to confirm the association of this genetic marker with allergic rhinitis or atopy, the measurement of the blood ECP levels would be added for determining the effect of the RNase3 gene polymorphism.
Larger population-based studies are necessary to confirm the association of genetic markers with allergic rhinitis or atopy. The Swedish genetic association study was a case–control study of 209 controls and 76 asthmatic subjects and the other Swedish genetic association study was 163 and 151, respectively [2, 3]. The Norwegian association study included 177 families with asthmatic probands [8]. The present study was a case–control study of 478 controls and 440 allergic rhinitis subjects.
The prevalence of the g.371G>C and g.499G>C polymorphisms in both the controls and patients with allergic rhinitis in our study differ from the prevalences reported in the Swedish [2, 3] and Norwegian [8] studies. The difference between previous studies and our study appears to reflect the different study populations. Specifically, the previous studies involved Scandinavian populations, whereas our study involved a Korean population. Previous studies have suggested that there are significant interethnic differences in the allele frequencies of allergic rhinitis, with predisposition genotypes according to the races [4–6]. The ethnic background may be important.
Future studies in allergic rhinitis are required using proper study design with adequate statistics, haplotyping of polymorphisms, and relevant phenotypes. In genetic association studies, such as the present study, functional studies should be done for determining the effect of the gene polymorphisms. Therefore, SNPs studies would provide high-level evidence for the interaction between genes, environment, and disease expression in allergic rhinitis, confirming the association of this genetic marker with allergic rhinitis or atopy.
Conclusion
The g.499G>C polymorphism in the ECP gene may be susceptible to the development of allergic rhinitis. The present study also demonstrated a significant association between the G-C-G haplotype in the RNase3 gene and allergic rhinitis. According to our results, both the haplotype and SNP effect could potentially influence the development of allergic rhinitis.
Further functional studies of RNase3 genetics in light of the associations with allergic rhinitis inflammation would be needed for determining the effect of the RNase3 gene polymorphism.
References
Amin K, Ludviksdottir D, Janson C, Nettelbladt O, Bjornsson E, Roomans GM, Boman G, Seveus L, Venqe P (2000) Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. Am J Respir Crit Care Med 162:2295–2301
Jonsson UB, Bystrom J, Stalenheim G, Venge P (2002) Polymorphism of the eosinophil cationic protein-gene is related to the expression of allergic symptoms. Clin Exp Allergy 32:1092–1095
Jonsson UB, Bystrom J, Stalenheim G, Venge P (2006) A (G→C) transversion in the 3′ UTR of the human ECP (eosinophil cationic protein) gene correlates to the cellular content of ECP. J Leukoc Biol 79:846–851
Kim JJ, Lee JH, Jang CH, Kim YS, Chae SC, Chung HT, Choi TW, Lee JH (2004) Chemokine RANTES promoter polymorphisms in allergic rhinitis. Laryngoscope 114:666–669
Kim JJ, Kim HJ, Lee KI, Chung HT, Lee JH (2004) Association between polymorphisms of the angiotensin-converting enzyme and angiotensinogen genes and allergic rhinitis in a Korean population. Ann Otol Rhinol Laryngol 113:297–302
Kim JJ, Min JY, Lee JH (2007) Polymorphisms in the IL-13 and IL-4 receptor alpha genes and allergic rhinitis. Eur Arch Otorhinolaryngol 264:395–399
Martin LB, Kita H, Leiferman KM, Gleich GJ (1996) Eosinophils in allergy: role in disease, degranulation and cytokines. Int Arch Allergy Immunol 109:207–215
Munthe-Kaas MC, Gerritsen J, Carlsen KH, Undlien D, Egeland T, Skinningsrud B, Torres T, Carlsen KL (2007) Eosinophil cationic protein (ECP) polymorphisms and association with asthma, s-ECP levels and related phenotypes. Allergy 62:429–436
Noguchi E, Iwama A, Takeda K, Takeda T, Kamioka M, Ichikawa K, Akeba T, Arinami T, Shibasaki M (2003) The promoter polymorphism in the eosinophil cationic protein gene and its influence on the serum eosinophil cationic protein level. Am J Respir Crit Care Med 167:180–184
Rosenberg HF, Domachowske JB (1995) Rapid evolution of a unique family of primate ribonuclease genes. Nat Genet 10:219–223
Rosenberg HF, Domachowske JB (2001) Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol 70:691–698
Acknowledgments
This paper was supported in part by a Soongsan fellowship in Wonkwang University in 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, I., An, Xh., Oh, YK. et al. Identification of polymorphisms in the RNase3 gene and the association with allergic rhinitis. Eur Arch Otorhinolaryngol 267, 391–395 (2010). https://doi.org/10.1007/s00405-009-1103-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-009-1103-8