Abstract
Idiopathic sudden hearing loss (ISHL) has been suggested to precipitate as final common pathway of microcirculatory impairment of the inner ear associated with a variety of etiologies and characterized by a local hyperviscosity syndrome in cochlear vessels. Therefore, we investigated the effect of Rheopheresis, a method of therapeutic apheresis reducing plasma viscosity and improving microcirculation on hearing recovery. Patients were randomly assigned to receive two Rheopheresis treatments, or treatment according to current German guidelines consisting either of i.v. corticosteroids (methylprednisolon 250 mg for 3 days and subsequent oral dosing with tapering to zero) or i.v. hemodilution (500 mL 6% hydroxyethyl starch plus 600 mg pentoxifylline per day), each applied for 10 days. The primary outcome parameter was absolute recovery of hearing as measured by pure tone audiometry 10 days after the start of treatment. Secondary outcomes were recovery of hearing at day 42, the improvement of speech audiometry, tinnitus and feeling of pressure and the frequency of adverse events. In total, 240 patients with sudden hearing loss were enrolled from otorhinolaryngological departments at hospitals as well as out-patient clinics in Germany. Analysis was performed for the intention-to-treat as well as per protocol population. Mean absolute recovery of hearing on day 10 within the intention-to-treat population (ITT, n = 193) was 23.95 dB (SD 15.05) in the Rheopheresis group and 24.29 dB (SD 15.48) in the control group. Equal efficacy of Rheopheresis and tested standard treatments was demonstrated (P = 0.00056). Single Rheopheresis led to a higher recovery of hearing after 48 h in patients with high plasma viscosity (>1.8 mPas s; P = 0.029) or high total protein (>74 g/dL; P = 0.02). However, an overall good recovery of ISHL was observed with none of the tested therapies being superior regarding the primary outcome parameter. Improvement of health-related quality of life as documented by the SF36 was higher in the Rheopheresis group, exhibiting a significant difference for the physical summary scale at the final follow-up at day 42 (P = 0.006). In conclusion, Rheopheresis proved to be an effective treatment option within the ENT armamentarium for ISHL. Two Rheopheresis treatments within 3 days lasting for about 2 h each could be used to replace a 10-day infusion regimen, especially in patients who desire fast recovery from acute hearing loss. Also, this may be a second line treatment option for patients refractory to i.v. corticosteroids or hemodilution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Investigations on idiopathic sudden hearing loss (ISHL) have yielded a wide range of evidence over the last few decades regarding underlying etiologies, pathogenic mechanisms, and therapeutic approaches [1–7]. Prognostic evaluation for the individual patient remains to be difficult due to the variety of etiologies [8–10]. Vascular pathogenesis of ISHL has been discussed in numerous studies, most of them arguing that the suddenness of the event and the anatomical architecture of the cochlear vasculature, with the labyrinthine artery being a functional end artery, represent a vulnerable microcirculatory environment very likely to be affected by vascular events as final common pathway [11, 12] (see Fig. 1). Some authors suggested thrombophilic vascular involvement in the pathogenesis of ISHL and recommended that all patients should undergo a comprehensive hematologic investigation of inherited and acquired prothrombotic factors [13]. In particular, these factors might be correlated with a high risk of recurring hearing loss. A hypercoaguable state has been proposed to be involved in the suggested local hyperviscosity syndrome, causing an impairment of cochlear microcirculation [14, 15]. Altered blood flow characteristics, a reduction of arterial or venous blood flow, impaired local vascular regulation or changes in the texture of the vessel wall represent pathogenic mechanisms for ISHL in this context [16, 17]. Autoimmune diseases and virus infections were also discussed as underlying etiologies of ISHL in recent years [18].
These various etiologies are assumed to lead to a final common pathophysiologic pathway ultimately impairing cochlear blood flow, and reducing it to a level insufficient to maintain cochlear function. Maass [17] pointed out that elevated blood viscosity and coagulability due to an increase in high-molecular weight proteins is a likely pathogenic factor of ISHL. Full blood and plasma viscosity have been shown to correlate with the extent of hearing loss in ISHL [19]. Fibrinogen as a major determinator of plasma viscosity in particular is related to ISHL [20]. Higher levels of fibrinogen or cholesterol were related to improved hearing recovery in ISHL patients after treatment with fibrinogen–LDL-apheresis, covering Rheopheresis and HELP-apheresis (heparin-induced extracorporeal LDL precipitation) for the indication of ISHL [21]. Rheopheresis treatment simultaneously eliminates an exactly defined spectrum of high-molecular weight (>50 kDa), rheologically relevant plasma proteins (i.e., alpha-2-macroglobulin, fibrinogen, LDL-cholesterol, von Willebrand factor (vWF), IgM, fibronectin, and putatively multimeric vitronectin) [22, 23]. HELP-apheresis originally was developed for extracorporeal treatment of severe hypercholesterolemia but additionally eliminates fibrinogen and a broad spectrum of clotting factors and coagulation regulators [24]. Also positive effects of pharmaceutical defibrinogenation therapy are in accord with these considerations [25].
In this study Rheopheresis was compared to current standard treatments with corticosteroids and hemodilution, respectively, in a prospective, randomized, controlled study design. Treatment of ISHL with systemic steroids and hemodilution using hydroxyethyl starch represent cornerstones of current therapy regimens in Northern American and European countries, although a final consensus on efficacy remains to be concluded [26, 27]. Rheopheresis has been shown to lower full blood and plasma viscosity, as well as platelet aggregation by elimination of high-molecular weight proteins, effectively, using a double-filtration process [22]. Improvement of cochlear perfusion and microcirculation on a functional level can be hypothesized to be the result of this therapeutic approach.
Methods
Patients, treatments, and investigations
Patients were recruited consecutively starting in the year 2000 and lasting approx. 5 years from otorhinolaryngological departments at hospitals (n = 6, with three university hospitals), as well as out-patient clinics and practices (n = 36) in Germany sending the patients to a total of 18 nephrological departments at hospitals (n = 6, with three university department) and out-patient clinics and practices (n = 12) being experienced in performing ambulatory therapeutic apheresis in particular double filtration plasmapheresis on a routine basis. Patients were allocated to either Rheopheresis treatment group or to one of the standard control groups, corticosteroids or hemodilution. Rheopheresis treatment consisted of two sessions performed within 2–3 days at the locally cooperating apheresis center. In case the patient achieved a remission after the first Rheopheresis the second treatment was omitted. Corticosteroids as well as hemodilution were applied during a 10-day out-patient infusion therapy at the discretion of the investigator (methylprednisolone i.v. 250 mg/day for 3 days, followed by an oral tapering to 0 over 7 days or hydroxyethyl starch 6% 500 mL/day plus pentoxifylline 600 mg/day for 10 days, respectively). Within the study group there was a consensus not to include a sham-apheresis placebo group due to the general expectation of ISHL patients in Germany to receive an active treatment. A placebo control group would have created an insuperable hurdle for enrolling patients.
One individual randomization list for each center with a block size of six was generated, that was not disclosed to the investigators. Whenever a patient was eligible for inclusion into the trial and had signed the informed consent document, the study sites contacted the office of the principle investigator, who provided the patient assignment to Rheopheresis or control group, (i.e., either corticosteroids or hemodilution treatment) according to the sequence of the randomization list as prepared by the statisticians.
All patients were examined by the otorhinolaryngological investigator at study sites to validate inclusion criteria before enrollment. During the course of the study the otorhinolaryngologist examined the patient and conducted pure tone audiograms on day 1, 2, 5, 10, and 42 (follow-up). Speech discrimination was recorded on day 1, 5, 10, and 42 using the Freiburger speech discrimination test (DIN 45621; monosyllabic numbers, [28]). Following a modified recommendation of German guidelines for the treatment of ISHL [29] advising against this speech discrimination test in early ISHL, it was not carried out anymore after interim analysis. A nephrologist before randomization examined patients regarding general exclusion criteria. Concomitant symptoms of ISHL tinnitus and feeling of pressure, medication, and medical history were documented prior to the first treatment. Subsequent changes were documented at the respective visit. All patients documented subjectively perceived health-related quality of life at the initial visit and days 10 and 42 using the standardized SF36 questionnaire. Patients and investigators were not blinded due to the considerable differences between treatment modalities. Prior to the commencement of the study, ethical approval had been obtained for every study site and notification was done to the appropriate federal state and institutional authorities. Each participant gave written informed consent before initiation of any study-related activities.
Inclusion and exclusion criteria for patients
Patients aged 18–75 whose symptoms had started less than 7 days prior to the first treatment were included and randomized into one of the treatment arms. ISHL was defined as a sudden loss of at least 30 dB in two frequencies of the main speech range (250–4,000 Hz) compared to the unaffected ear or previously recorded pure tone audiograms of the affected ear. The frequencies 250, 500, 1,000, 1,500, 2,000, 3,000, and 4,000 Hz were tested. The threshold of hearing loss was changed from 30 to 20 dB by an amendment after interim analysis at the beginning of the second part of the study due to very slow patient enrollment. It was a requirement that hearing of the contra-lateral ear was not compromised. Patients were not included if they met any of the following otologic exclusion criteria: pre- or post-cochlear damage (i.e., hearing loss due to causes in the external ear canal and/or the middle ear or hearing loss due to pathologies involving the acoustic nerve and central structures), evidence of an acute viral or bacterial infection, complete deafness (by definition: pantonal hearing loss >80 dB), bilateral acute hearing loss, the presence of Menière’s Disease (inner ear disorder that can affect both hearing and balance with episodes of vertigo, hearing loss, tinnitus, and the sensation of fullness in the ear), or recurrence of an anamnestically pre-existing acute hearing loss during the past 12 months. Any type of intravenous pre-treatment with the purpose of improving acute hearing loss (i.e., HES or other plasma expanders, steroids, and vasodilators) was not permitted. For oral pre-treatment low-dose acetylsalicylic acid, pentoxifyllin, naftdrofuryl, low-dose oral steroid (methyl-prednisolone <20 mg/day) for up to 3 days prior to the start of study treatment was allowed. Treatment with ototoxic medications (e.g., acetylsalicylic acid >500 mg/day, amino glycosides, high-dose furosemide, cytostatic drugs) within the past 14 days was not permitted. Furthermore, the following general exclusion criteria were defined mainly representing general aspects for the performance of out-patient therapeutic apheresis: anemia, hemorrhagic diathesis, coagulopathy, unstable hemodynamics which did not allow the use of extracorporeal circulation (unstable angina pectoris, coronary heart disease in stage CCS III–IV, chronic heart failure in stage NYHA III–IV, symptomatic arrhythmia), hypotonia with systolic blood pressure below 100 mmHg, severe kidney or liver malfunctions, chronic viral infections (e.g., hepatitis B or C, HIV), pregnancy in women of childbearing age, lactating women, epilepsy, psychosis or dementia, manifest malignant disease, alcohol or drug abuse, nicotine abuse (>20 cigarettes/day), simultaneous participation in another clinical investigation, vein conditions which did not allow peripheral venous access for performing Rheopheresis, therapy with anticoagulant medications (e.g., heparin s.c. for thrombosis prophylaxis or coumarin preparations), and patients with anamnestically known heparin-induced thrombocytopenia. Standard exclusion criteria for the administration of glucocorticoids were gastro-intestinal ulcers, severe osteoporosis, infections with pathogenic germs, time period of up to 4 weeks following prophylactic immunizations, narrow-angle and wide-angle glaucoma, insufficiently controlled diabetes mellitus, and proven tuberculosis within the past 2 years.
Rheopheresis treatment
Rheopheresis is an application of double filtration plasmapheresis (DFPP) specifically designed to treat microcirculatory disorders using a polyethylene membrane plasma separator and a Rheofilter for plasma filtration in combination with an appropriately controlled pump technology as described below. One Rheopheresis treatment simultaneously eliminates an exactly defined spectrum of high-molecular weight (>50 kDa), rheologically relevant plasma proteins [22, 23]. Rheopheresis was performed ambulatory at the treatment centers using the Octo Nova device (SW 4.27) combined with Octo Therm heating system and tubing system MF-430 (Diamed Medizintechnik, Cologne, Germany). Plasma was separated with the polyethylene plasma filter OP-05 W (Asahi Kasei Kuraray Medical, Tokyo, Japan) with a continuous blood flow of 60−90 mL/min. Blood cells were directly returned to the patient. Elimination of plasma proteins was achieved with plasma filtration using the Rheofilter (effective surface of 1.7 m2; Asahi Kasei Kuraray Medical, Tokyo, Japan) with a plasma flow of 10–30 mL/min. Vascular access was established by puncture of peripheral cubital or forearm veins. Target was to treat 100% of patients’ plasma volume per Rheopheresis session. Anticoagulation was performed with unfractionated heparin (upto 5,000 IE initial bolus followed by 1,000–2,500 IE/h continuously). Foreign blood or plasma components were not substituted with the procedure. The complete set for Rheopheresis consists solely of disposable products. Both the Rheofilter and the plasma separator Plasmaflo OP-05 are CE-certified in accordance with European regulations for medical devices. Rheopheresis is a trademark of Diamed Medizintechnik, Cologne, Germany.
Study design and statistical analysis
The primary outcome parameter was the absolute recovery of hearing in dB as determined by the pure tone audiogram conducted upon the final examination on day 10 in comparison to the initial examination. Remission was defined as a hearing recovery to ±5 dB in comparison to the contra-lateral, presumably unaffected ear, considering the age-related thresholds for the contra-lateral ear. Recovery meant a threshold that was in the average of all affected frequencies not more than 5 dB less than that of the unaffected side (regarding these frequencies). Secondary endpoints were defined as the absolute recovery of hearing on days 2, 5, and 42. Furthermore, relative recovery of hearing and the recovery from concomitant symptoms such as tinnitus and the feeling of pressure in the affected ear on days 2, 5, 10, and 42. Recovery of speech perception was measured on days 5, 10, and 42.
The relative recovery of hearing was calculated as a quotient from the absolute recovery of hearing at the particular time of interest and the absolute hearing loss at the initial examination. As Rheopheresis was compared to two standard treatment options—corticosteroids and hemodilution—the absolute and relative recovery of hearing also were analyzed separately for each group.
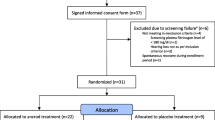
A two-step sequential adaptative analysis was chosen for the study, with a one-sided level α = 0.05, based on a Pocock design with adapted critical levels of α = 0.031. This statistical approach was chosen with respect to the, at the time of the design of the trial, unknown effect size of this new therapeutic procedure. The adaptive design was chosen to reserve more statistical power for the final test. Analysis of the pooled data without alpha-correction was performed by a weighted two-way variance t test. An interim analysis was performed after inclusion of the first 60 patients, referred to as part I (Rheopheresis: 30 patients; corticosteroids and hemodilution: 30 patients). Assuming non-inferiority of rheopheresis to be investigated, the necessary sample size for part I of the study was estimated to be 60. The threshold of non-inferiority was defined as 8 dB. In the event of significance, the study would have been discontinued with a positive result. Thus, the power in part I amounts to 1 − β = 0.8 assuming a standardized difference Δ/δ = 1. Based on the results of the interim analysis, further 180 patients were to be enrolled into the study, referred to as part II, until the recalculated final sample size of 240 participants was reached. The P values of both parts I and II were combined with the inverse-normal method and likewise assessed on the basis of α = 0.031. Patients who fulfilled the inclusion criteria for initial hearing loss, received therapy, and have documented at least one follow-up observation of the primary outcome were analyzed within the intention-to-treat (ITT) population. Patients who met all inclusion criteria and have documented all follow-up observations of the primary outcome were analyzed within the per protocol (PP) population. Missing values were replaced with a last-value option. The study flow chart is depicted in Fig. 2.
Raw data lists were made from all recorded data. The data were portrayed descriptively and statistically by suitable statistical variables of central tendency (mean value, median), dispersion (standard deviation, quartile, minima and maxima) or frequencies, divided according to measuring time points.
Results
In total, 240 patients were enrolled in this prospective, multicenter, randomized, controlled clinical trial. A total of 222 patients could be assessed for safety, 193 as ITT (107 male, 86 female, mean age 45.3 years) and 161 as PP population. Almost all patients randomized to the Rheopheresis group received two treatments with a mean number of 1.96 and a mean treatment volume of 2,800 mL per session. Demographic and baseline characteristics are described in Table 1. Analysis of the ITT population regarding the primary outcome with respect to equal efficacy of Rheopheresis revealed no significant difference. The primary outcome calculated and analyzed within the PP population also demonstrated equal efficacy of Rheopheresis compared to standard treatment (intravenous hemodilution or corticosteroids) with respect to the absolute recovery of hearing after a full course of therapy on day 10 (P = 0.00056). In part I, n 1 = 57 patients were included in the PP set. The one-sided P value of the primary endpoint in part I was p 1 = 0.0035. In part II, n 2 = 104 patients were included in the PP set. No significant differences between parts I and II could be detected for any study visit, proving poolability of all patients for final analysis. The one-sided P value of the primary endpoint in part II was p 2 = 0.014. According to the Φ−1-rule, the combined p-value was ϕ \( \left[ {{{\left( {\Phi^{ - 1} (p_{1} )} \right) + \left( {\Phi^{ - 1} (p_{2} )} \right)} \mathord{\left/ {\vphantom {{\left( {\Phi^{ - 1} (p_{1} )} \right) + \left( {\Phi^{ - 1} (p_{2} )} \right)} {\sqrt 2 }}} \right. \kern-\nulldelimiterspace} {\sqrt 2 }}} \right] = 0.000 5 6 \). This was smaller than the critical Pocock adjusted α = 0.031. Therefore, the null hypothesis stating Rheopheresis to be the inferior therapy, could be rejected with a one-sided level α = 0.05 in this trial. All further final analyses were carried out within the ITT population. A slight numeric difference in the absolute recovery of hearing was observed at day 2 in favor of Rheopheresis in the ITT population with patients having regained more of their hearing loss compared to standard treatment (mean difference 2.57 dB). However, this difference was not statistically significant and was not maintained at later visits (see Fig. 3). Relative recovery of hearing within the ITT population in the control group was 77 versus 76% for the Rheopheresis group at day 10 and 86 versus 82% at day 42 without a significant difference (Table 2).
Comparison of initial loss and recovery of hearing of the Rheopheresis group with the hemodilution group, the corticosteroid group, and the pooled control group (hemodilution + corticosteroids), respectively, did not reveal significant differences within the final analysis. Comparison of both control groups showed a significantly higher initial loss and recovery of hearing in the HES control group. The presence of these two control groups in this trial is related to a change of German guidelines for the treatment of ISHL [29] and, in consequence, a changed standard of care. In the beginning of the trial, this standard consisted of an in-hospital treatment with hemodilution therapy. This changed to an out-patient therapy using either i.v. prednisolone or hemodilution at the discretion of the physician with steroids being more in favor. Consequently, patients who were randomized to the control group in the first part of this study were exclusively treated with hemodilution, while in the second part mainly steroids were applied. Additionally, the threshold of hearing loss in the inclusion criteria was changed from 30 to 20 dB at the beginning of the second part of the study due to very slow patient enrollment. This led to an inclusion of patients meeting all the inclusion criteria but with less pronounced ISHL. In summary, this resulted in a certain disparity of the initial loss of hearing in the control group and a higher overall recovery within the hemodilution group. However, no significant differences were found when analyzing the relative recovery of hearing at both days 10 and 42. When analyzing subgroups of patients with severe initial loss of hearing (>40 dB in at least two frequencies), no significant differences could be found in the absolute recovery of hearing. Patients whose hearing loss did not improve beyond a hearing gain of 5 dB, or whose hearing loss worsened were also less likely to recover from tinnitus either.
No statistically significant differences were found between the Rheopheresis and the control group regarding recovery of speech perception and the recovery from concomitant symptoms such as tinnitus and the feeling of pressure in the affected ear. Rheopheresis led to faster recovery, whereby a larger proportion had recovered from tinnitus and from the feeling of pressure at day 10 compared to the control group (Table 2). Remission was defined as loss of hearing ≤5 dB compared to the contra-lateral, healthy ear. Proportions of patients in the control and Rheopheresis groups, who could be classified as “in remission” according to this very tight definition at both days 10 and 42 were 47 versus 36% and 52 versus 47% within the ITT population. None of these differences were statistically significant.
Rheopheresis led to an efficient reduction of hemorheological parameters. Comparison of pre- and post-apheresis values of fibrinogen, LDL-cholesterol, and α2-macroglobulin showed a significant decrease. When analyzing subgroups separated by the median of cholesterol, LDL-cholesterol and fibrinogen with respect to their absolute recovery of hearing and speech perception did not yield significant differences. However, patients with high plasma viscosity (>1.8 mPas s; P = 0.029) or high normal range of total plasma protein (>74 g/dl; P = 0.02) had recovered significantly more from their lost hearing after the first Rheopheresis on day 2. Absolute recovery of hearing was still higher on days 10 and 42, but no longer statistically significant.
In terms of health-related quality of life, a statistically significant difference in favor of Rheopheresis could be found with respect to the physical health main scale at day 42, at which time point the Rheopheresis group scored higher (Fig. 4). Overall, Rheopheresis patients scored higher on all subscales at day 42 (significantly so on the bodily pain and physical role function subscale), indicating a higher perceived quality of life regarding these aspects. Within each treatment group, quality of life improved significantly from the initial visit to day 42, and scores on physical subscales in the Rheopheresis group at day 42 were higher compared to a large representative sample of the German general population [30].
Safety was assessed by analyzing physical data, laboratory results, and adverse events. Ten adverse events were documented in total. Two of these adverse events were marked as serious and consisted of nausea, vomiting and the suspicion of cerebral or metabolically associated seizures putatively caused by a severe allergic reaction, occurring in one patient within the Rheopheresis group. During the subsequent course the patient recovered completely and notably had remission of ISHL. A similar event has never been observed with any Rheopheresis treatment for any indication. Eight of the documented events, e.g., transient hypotension or dizziness, occurred in the Rheopheresis group and two in the control group. No patterns as to patient characteristics predisposing certain individuals to adverse events or increased vulnerability among the investigated patients could be identified. No particular group of patients emerged from the safety evaluation as being more at risk for experiencing adverse events. This favorable safety profile was in accord with the experience on fibrinogen–LDL-apheresis in another randomized trial investigating an essentially identical ISHL patient population [21].
Discussion
The German multicenter trial on Rheopheresis presented here was one of the largest controlled prospective, randomized multicenter trials successfully completed in the field of ISHL. A recent comprehensive review of all ISHL randomized controlled trials [2, 3] revealed only three further studies with populations larger than 150 participants [21, 31, 32], with a mean of 76 enrolled patients for all 20 analyzed trials. With this study there are now two randomized controlled trials on fibrinogen–LDL-apheresis including each more than 200 patients. Fibrinogen–LDL-apheresis in this context of ISHL includes Rheopheresis and HELP-apheresis [21–23].
The equal efficacy of Rheopheresis compared to two modalities of standard ISHL treatment was proven with statistical significance. In the final analysis advantages could be observed in the areas of early recovery as previously reported for fibrinogen–LDL-apheresis [21] and quality of life after 6 weeks. In conclusion, Rheopheresis is a safe and highly effective treatment option. Patients with high plasma viscosity or high normal range of total plasma protein had a significantly higher benefit at day 2. Safety and efficacy of fibrinogen–LDL-apheresis as a treatment option for ISHL could be confirmed. Patients within the control groups of this trial recovered very well confirming efficacy of current standard treatments with i.v. hemodilution or i.v. corticosteroids [5, 33]. Fibrinogen–LDL-apheresis performed as HELP-apheresis and including the above-mentioned randomized trial has been shown [20, 21] to be equally effective to a treatment regimen consisting of hydroxyethyl starch, pentoxifylline, and steroids. No difference could be shown for pure tone audiometry between the apheresis group and the control group at day 42, which is in complete accord with the results presented here. Within the fibrinogen–LDL-apheresis group, patients above the median of fibrinogen in this patient population seemed to benefit more [21].
Comparing the pre- and post-interim analysis sections, patients included in the second part of this study tended to have a lower initial loss of hearing compared to participants in the first part of the study. A slight trend for Rheopheresis being more effective compared to standard treatment, as observed in the first part of the study, could not be reproduced with the less severely affected ISHL patients in the second part. Efficacy differences have been leveled out. From a statistical point of view, it has been argued that patients with an average hearing loss of at least 40–60 dB need to be included in studies intending to show a difference between two effective treatments. This measure would allow for an average absolute hearing recovery rate large enough to show a statistical difference [34].
The results of both controlled randomized clinical studies on fibrinogen–LDL-apheresis indicate, that a group of patients characterized by the common features of high plasma viscosity, a high normal range of total plasma protein, LDL–cholesterol, or fibrinogen has to be discussed as a target for apheresis procedures, arguing that the presence of (micro-) vascular risk factors determines to a certain extent the benefit from Rheopheresis compared to an non-stratified sample as recruited in this study [21, 22]. However, enrolling such a subgroup of patients would be a very difficult task. Currently, plasma parameters can be regarded as prognostic factors, proving the concept, but not as markers for the individual patient to decide on the use of fibrinogen–LDL-apheresis. As this study’s results were analyzed with regard to serological parameters established as (micro)-vascular risk factors (e.g., fibrinogen, cholesterol, and LDL–cholesterol), and no significant differences could be found, one could argue that further stratification (e.g., age) is necessary in order to identify a subgroup of patients likely to benefit from Rheopheresis. Conversely, the question arises whether differences with regard to recovery of hearing between the investigated treatments—Rheopheresis, hemodilution, and corticosteroid infusions—are large enough to be detected.
Rheopheresis treatments effect the immediate pulsed reduction of plasma viscosity as well as whole blood viscosity, which has been proven significantly in this trial. The therapy can lead to a sustained microcirculatory recovery, and may change significantly the natural course of acute microcirculatory impairment [22, 35]. Microcirculation also consists of rheological, functional, and structural aspects and comprises the entire interactive network of the blood vessel system with the surrounding tissue on the cellular and molecular level. In addition to the vascular and haemorheological effects of apheresis, recent pathogenetic findings indicate a further putative mechanism of action. The direct reduction of plasma cholesterol concentrations could also result in a modulation of the perilymph compartment and the composition of outer hair cell (OHC) membranes in particular the ratio of phospholipids to cholesterol. In vitro experiments demonstrated that the lateral wall of OHCs from guinea pig cochlea can incorporate water-soluble cholesterol and may interact with the OHC’s membrane directly [35]. It is supposed that this may lead to an increased stiffness of the cells and impair the electro motile response of OHCs. The fast motility of the OHCs, physiologically essential for cochlear amplification of low sound intensities, could be disturbed by the increased stiffness. Recent findings indicate that alterations in membrane cholesterol also affect the function of 80-kDa integral membrane protein prestin, a critical component of OHC lateral wall essential for electro motility, and functionally tune the outer hair cell [37]. Hyperlipidemia and atherosclerosis can induce alterations in cochlear morphology and function in mice [38]. Consequently, a pulsed reduction of plasma lipoproteins by Rheopheresis might result in an efflux of these molecules from OHC membranes, leading to a decrease of stiffness and improved electro motility. The potential interrelationship of plasma and OHC membrane cholesterol being in a dynamic equilibrium might be positively influenced by pulsed Rheopheresis treatment [36–38].
Quality of life was documented to be higher at day 42 in the Rheopheresis group, even compared to the German normal population on physical health and quality of life subscales. Whether this is related to Rheopheresis being administered less often compared to daily infusions, or subjectively perceived better improvement of hearing, cannot be concluded from the SF36 data. Treatments leading to accelerated recovery in combination with a minimal number of therapeutic interventions like Rheopheresis seemed to have a considerable effect on quality of life. These criteria could provide additional information when choosing the most appropriate treatment option.
However, as the ISHL population is very diverse, certain subgroups might benefit more from Rheopheresis than others. Further research needs to establish these subgroups of ISHL patients. Up to date, fibrinogen–LDL-apheresis has been shown to be equally effective compared to standard polypragmatic infusion therapy of patients for the indication of first onset ISHL in two large controlled, randomized clinical trials, including this study, and the trial conducted by Suckfüll et al. [21]. In patients with recurrent ISHL, in particular if associated with progressive and persisting severe hearing loss, standard infusion therapy often results in unsatisfactory outcome. Recent results of a retrospective analysis of 25 ISHL patients showed that Rheopheresis could improve restoration of hearing of recurring ISHL in patients refractory to polypragmatic infusion therapy, while hearing loss remained almost unchanged during standard therapy for acute events, 68% of patients showed immediate improvement or recovery of hearing after two consecutive Rheopheresis treatments (S. Uygun-Kiehne, personal communication).
In another recent retrospective case series fibrinogen–LDL-apheresis achieved complete or partial remission in 54% of patients even after unsuccessful treatment with another therapy [39]. Fibrinogen–LDL-apheresis namely Rheopheresis in this trial has been established as an effective treatment option for ISHL thus increasing the therapeutic armamentarium also for patients refractory to the first-line standard treatment. This treatment schedule translates German ISHL guidelines into practice, proposing fibrinogen-lowering treatments like fibrinogen–LDL-apheresis as part of a multimodality approach.
References
Koc A, Sanisoglu O (2003) Sudden sensorineural hearing loss: literature review on recent studies. J Otolaryngol 32(5):308–313. doi:10.2310/7070.2003.11288
Conlin AE, Parnes LS (2007) Treatment of sudden sensorineural hearing loss I—a systematic review. Arch Otolaryngol Head Neck Surg 133:573–581. doi:10.1001/archotol.133.6.573
Conlin AE, Parnes LS (2007) Treatment of sudden sensorineural hearing loss II—a meta-analysis. Arch Otolaryngol Head Neck Surg 133:582–586. doi:10.1001/archotol.133.6.582
Domachevsky L, Keynan Y, Shupak A, Adir Y (2007) Hyperbaric oxygen in the treatment of sudden deafness. Eur Arch Otorhinolaryngol 264(8):951–953. doi:10.1007/s00405-007-0283-3
Alles MJ, der Gaag MA, Stokroos RJ (2006) Intratympanic steroid therapy for inner ear diseases, a review of the literature. Eur Arch Otorhinolaryngol 263(9):791–797. doi:10.1007/s00405-006-0065-3
Burschka MA, Hassan HA, Reineke T, van Bebber L, Caird DM, Mösges R (2001) Effect of treatment with Ginkgo biloba extract EGb 761 (oral) on unilateral idiopathic sudden hearing loss in a prospective randomized double-blind study of 106 outpatients. Eur Arch Otorhinolaryngol 258(5):213–219. doi:10.1007/s004050100343
Fujimura T, Suzuki H, Shiomori T, Udaka T, Mori T (2007) Hyperbaric oxygen and steroid therapy for idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol 264(8):861–866. doi:10.1007/s00405-007-0272-6
Cadoni G, Scipione S, Rocca B, Agostino S, La Greca C, Bonvissuto D, Paludetti G (2006) Lack of association between inherited thrombophilic risk factors and idiopathic sudden sensorineural hearing loss in Italian patients. Ann Otol Rhinol Laryngol 115(3):195–200
Ceylan A, Celenk F, Kemaloglu YK, Bayazit YA, Göksu N, Özbilen S (2007) Impact of prognostic factors on recovery from sudden hearing loss. J Laryngol Otol 121:1035–1040. doi:10.1017/S0022215107005683
Hoth S (2005) On a possible prognostic value of otoacoustic emissions: a study on patients with sudden hearing loss. Eur Arch Otorhinolaryngol 262(3):217–224. doi:10.1007/s00405-004-0797-x
Selmani Z, Pyykko I, Ishizaki H, Marttila TI (2001) Cochlear blood flow measurement in patients with Meniere’s disease and other inner ear disorders. Acta Otolaryngol Suppl 545:10–13
Hirano K, Ikeda K, Kawase T, Oshima T, Kekehata S, Takahashi S, Sato T, Kobayashi T, Takasaka T (1999) Prognosis of sudden deafness with special reference to risk factors of microvascular pathology. Auris Nasus Larynx 26(2):111–115. doi:10.1016/S0385-8146(98)00072-8
Capaccio P, Ottavani F, Cuccarini V, Bottero A, Schindler A, Cesana BM, Censuales S, Pignataro L (2007) Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope 117:547–551. doi:10.1097/MLG.0b013e31802f3c6a
Asakura M, Kato I, Takahashi K, Okada T, Minami S, Takeyama I, Ohnuki T (1995) Increased platelet aggregability in patients with vertigo, sudden deafness and facial palsy. Acta Otolaryngol Suppl 520(Pt 2):399–400. doi:10.3109/00016489509125281
Klemm E, Altmann E, Lange O (1983) Rheologische Probleme der Mikrozirkulation und Konsequenzen medikamentöser Hörsturztherapie. Laryngol Rhinol Otol (Stuttg) 62(2):62–64
Fowler EP Jr (1982) Intra-venule phenomena. Acta Otolaryngol 1961(53):107–115
Maass B (1982) Innenohrdurchblutung-Anatomisch-funktionelle Betrachtungen. HNO 30(10):355–364 Blood supply of the internal ear—anatomico-functional considerations
Lazarini PR, Camargo AC (2006) Idiopathic sensorineural hearing loss: etiopathogenic aspects. Rev Bras Otorrinolaringol (Engl Ed) 72(4):554–561
Ohinata Y, Makimoto K, Kawakami M, Haginomori S, Araki M, Takahashi H (1994) Blood viscosity and plasma viscosity in patients with sudden deafness. Acta Otolaryngol 114(6):601–607. doi:10.3109/00016489409126112
Suckfüll M, Thiery J, Wimmer C, Mees K, Schorn K (1997) Hypercholesterinämie und Hyperfibrinogenämie beim Hörsturz. Laryngorhinootologie 76(8):453–457 Hypercholesteremia and hyperfibrinogenemia in sudden deafness
Suckfüll M (2002) Fibrinogen and LDL apheresis in treatment of sudden hearing loss: a randomised multicentre trial. Lancet 360(9348):1811–1817. doi:10.1016/S0140-6736(02)11768-5
Klingel R, Mumme C, Fassbender T, Himmelsbach F, Altes U, Lotz J, Pohlmann T, Beyer J, Küstner E (2003) Rheopheresis in patients with ischemic diabetic foot syndrome: results of an open label prospective pilot trial. Ther Apher Dial 7(4):444–455. doi:10.1046/j.1526-0968.2003.00082.x
Klingel R, Fassbender C, Fassbender T, Göhlen B (2003) Clinical studies to implement Rheopheresis for age-related macular degeneration guided by evidence-based-medicine. Transfus Apheresis Sci 29(1):71–84. doi:10.1016/S1473-0502(03)00101-0
Jaeger BR, Goehring P, Schirmer J, Uhrig S, Lohse P, Kreuzer E, Reichart B, Seidel D (2001) Consistent lowering of clotting factors for the treatment of acute cardiovascular syndromes and hypercoagulability: a different pathophysiological approach. Ther Apher 5(4):252–259. doi:10.1046/j.1526-0968.2001.00350.x
Suzuki H, Furukawa M, Kumagai M, Takahasi E, Matsuura K, Katori Y, Shimomura A, Kobayashi T (2003) Defibrinogenation therapy for idiopathic sensorineural hearing loss in comparison with high-dose steroid therapy. Acta Otolaryngol 123(1):46–50. doi:10.1080/0036554021000028082
Nosrati-Zarenoe R, Arlinger S, Hultcrantz E (2007) Idiopathic sudden hearing sensorineural hearing loss: results drawn from the Swedish national database. Acta Otolaryngol 127:1168–1175. doi:10.1080/00016480701242477
Alexiou C, Arnold W, Fauser C, Schratzenstaller B, Gloddek B, Fuhrmann S, Lamm K (2001) Sudden sensorineural hearing loss: does application of glucocorticoids make sense? Arch Otolaryngol Head Neck Surg 127(3):253–258
DIN 45621-1, Freiburger Sprachtest, Sprache für Gehörprüfung-Teil 1: Ein-und mehrsilbige Wörter (1995) DIN 45621-2, Sprache für Gehörprüfung-Teil 2: Sätze, (1980)
Guideline for ISHL of the German Society for Otorhinolaryngology Head and Neck Surgery (Dt. Ges. f. HNO-Heilkunde KuH-C). (2004) AWMF guidelines No. 017/010. http://www.awmf.org
Bullinger M (1995) German translation and psychometric testing of the SF-36 health survey: preliminary results from the IQOLA project. International quality of life assessment. Soc Sci Med 41(10):1359–1366. doi:10.1016/0277-9536(95)00115-N
Kubo T, Matsunaga T, Asai H, Kawamoto K, Kusakari J, Nomura Y, Oda M, Yanagita N, Niwa H, Uemura T (1988) Efficacy of defribinogenation and steroid therapies on sudden deafness. Arch Otolaryngol Head Neck Surg 114(6):649–652
Probst R, Tschopp K, Ludin E, Kellerhals B, Podvinec M, Pfaltz CR (1992) A randomized, double-blind, placebo-controlled study of dextran/pentoxifylline medication in acute acoustic trauma and sudden hearing loss. Acta Otolaryngol 112:435–443. doi:10.3109/00016489209137424
Klemm E, Bepperling F, Burschka MA, Mösges R, Study Group (2007) Hemodilution therapy with hydroxyethyl starch solution (130/0.4) in unilateral idiopathic sudden sensorineural hearing loss: a dose-finding, double-blind, placebo-controlled, international multicenter trial with 210 patients. Otol Neurotol 28(2):157–170. doi:10.1097/01.mao.0000231502.54157.ad
Halpin C, Rauch SD (2006) Using audiometric thresholds and word recognition in a treatment study. Otol Neurotol 27(1):110–116. doi:10.1097/00129492-200601000-00020
Klingel R, Erdtracht B, Gauss V, Piazolo A, Mausfeld-Lafdhiya P, Diehm C (2005) Rheopheresis in patients with critical limb ischemia—results of an open label prospective pilot trial. Ther Apher 9(6):473–481. doi:10.1111/j.1744-9987.2005.00276.x
Nguyen TV, Brownell WE (1998) Contribution of membrane cholesterol to outer hair cell lateral wall stiffness. Otolaryngol Head Neck Surg 119:14–20. doi:10.1016/S0194-5998(98)70167-6
Rajagopalan L, Greeson JN, Xia A, Liu H, Sturm A, Raphael RM, Davidson AL, Oghalai JS, Pereira FA, Brownell WE (2007) Tuning of the outer hair cell motor by membrane cholesterol. J Biol Chem 282(5):36659–36670. doi:10.1074/jbc.M705078200
Guo Y, Zhang C, Du X, Nair U, Yoo TJ (2005) Morphological and functional alterations of the cochlea in apolipoprotein E gene deficient mice. Hear Res 208(1–2):54–67. doi:10.1016/j.heares.2005.05.010
Canis M, Heigl F, Hettich R, Osterkorn D, Osterkorn K, Suckfuell M (2008) H.E.L.P.-Apherese bei der Behandlung des Hörsturzes—Eine Anwendungsbeobachtung an 152 Patienten. HNO 9:961–966. doi:10.1007/s00106-008-1818-7
Acknowledgments
This study was supported financially by the Hans and Marlies Stock Foundation for Science and Research, Art and Culture, Cologne within the Stifterverband für die Deutsche Wissenschaft and Asahi Kasei Kuraray Medical Co., Ltd, Tokyo, Japan.
Study group
J. Floege, A. Nachtsheim, N. Pasch, M. Sondermann, Aachen; P. Tolsdorff, Bad Honneff; H. Scherer, W. Zidek, Berlin; K.A. Brensing, H. Wischerath, Bonn; A. Schadel, Darmstadt; J. Spaeth, B. Wölbert, Düren; J. Schlee, Eschweiler; W. Grotz, J. Lamprecht, Essen; T. Tsobanelis, W. von Heimburg, Frankfurt; P. Breitenberger, Germering; R. Budde, Haltern; R. Königsberger, Herrsching; H. Köhler, Homburg/Saar; P. Brendt, M. Lenzenhuber, W. Schütz, Jülich; H. Christ, B. Göhlen, A. Heibges, W. Lehmacher, U. Parpart, C·F. Peerenboom-Fey, T. Shahab, P. Schmalz, Cologne; S. Zymolka, Ludwigsfelde; U. Schirmböck, Ludwigshafen; H. Davids, H. Kingreen, H. Scholz, Lüdenscheid; K. Hörmann, A. Schwarzbeck, Mannheim; K. Frey, M. Schmidt, Marl; A. Goldmann, H. Seidler, Neunkirchen; K·H. Götz, I. Großmann, H. Künne, C. Mai, Neuruppin; K·H. Ahrens, V. Gläser, Plauen; G. Hartmann, Potsdam; T. Risler, H·P. Zenner, Tübingen; F·C. Burkart, Radolfzell; G. Schindlbeck, Viernheim.
Conflict of interest statement
Ralph Mösges has served as scientific advisor to Apheresis Research Institute and has received honoraria for lecturing. Reinhard Klingel received research funds from Diamed Medizintechnik GmbH, Cologne, Germany and Asahi Kasei Kuraray Medical Co., Ltd., Tokyo, Japan.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Mösges, R., Köberlein, J., Heibges, A. et al. Rheopheresis for idiopathic sudden hearing loss: results from a large prospective, multicenter, randomized, controlled clinical trial. Eur Arch Otorhinolaryngol 266, 943–953 (2009). https://doi.org/10.1007/s00405-008-0823-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-008-0823-5