Abstract
Despite several ways of investigation, such as clinical examination, drug-induced sleep endoscopy and pressure measurement of the upper airway, it is still difficult to locate the site of snoring exactly. Frequency analysis of snoring sounds is described as a promising diagnostic tool. The aim of the study was to examine simulated snoring under conditions awake, record the produced snoring sounds and compare those sounds with nocturnal snoring. A total of 50 snoring male patients were examined clinically by flexible nasal endoscopy and simulated snoring under conditions awake, and the simulated snoring sounds were recorded. Additionally, nocturnal snoring sounds were recorded during nighttime polysomnography. Snoring events were analyzed by fast-fourier-transformation and the intensity peaks 1–5 were evaluated. Rhythmic and non-rhythmic snoring events were distinguished depending on present obstructive apneas. Clinical and polysomnographical data were correlated with the results of the frequency analysis of the snoring sounds. Simulated snoring sounds revealed a low frequency of 200 Hz in intensity peaks 1 and 2 with an increase up to 3,000 Hz in peaks 3–5. Similar frequency patterns were detected in rhythmic nocturnal snoring. Non-rhythmic snoring events revealed frequency patterns between 2,000 and 3,000 Hz in all five intensity peaks. Simulated snoring resembles rhythmic nocturnal snoring with low-frequency intensity peaks, whereas non-rhythmic snoring revealed high frequencies. The examination during simulated snoring and frequency analysis of snoring sounds might contribute in locating the pathogenesis of snoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frequency analysis of snoring sounds has been reported as an additional diagnostic tool in combination with nighttime polysomnography in patients with suspected sleep-related breathing disorders [3, 8, 13, 14, 19, 21, 24]. Several studies reveal correlations between the frequency of snoring sounds and polysomnographic data like respiratory disturbance index (RDI) or apnea hypopnea index (AHI). Snoring sounds with a peak frequency above 400 Hz is reported by several authors to correlate with tongue base snoring, whereas snoring sounds with a peak frequency below 400 Hz are present in patients with primary snoring [2, 3, 14]. Despite this knowledge about the correlation between frequency of snoring sounds and sleep-related breathing disorders, the topodiagnosis of snoring remains still a major unsolved task. Apparently, the correlation between pathological results of a clinical examination of the upper airway and the extent of sleep-related breathing disorders is less than expected [11, 16, 22, 28]. The frequently propagated drug-induced sleep endoscopy provided questionable results in terms of comparability between drug-induced sleep and natural sleep [5, 10]. Concluding from literature data, a method that is sufficient to locate the origin of snoring does not exist yet. Nevertheless, flexible nasal endoscopy of the upper airway is an easy to perform and reliable method to obtain information about vibrating structures and sites of obstruction. A recently introduced technique of examination during simulated snoring under conditions awake has provided evidence for a correlation between the dislocation of anatomical structure during simulated snoring and the extent of sleep-related breathing disorders [16]. Thus, it appears reasonable to record the produced snoring sounds under simulated snoring as well as during nighttime polysomnography and compare both recordings. The aim of the study was to compare recorded snoring sounds, which are obtained awake and under sleep conditions to enable a topodiagnostic localization of snoring sounds.
Patients and methods
Clinical examination
A total of 50 male patients with a snoring history were included in the presented study. Inclusion criteria were reported snoring (not reported apneas), either by themselves or by the bed partners. The intention of asking for snoring instead of apneas was to obtain a heterogeneous spectrum including different types of snoring, which is not restricted to patients with suspected OSAS. Exclusion criteria was denial of nighttime snoring. All patients underwent an oto-rhino-laryngological examination of the upper airway (nasal septum, tonsil size, Malampatti index, dorsalization of the tongue base and Mueller Maneuver). Additionally, a flexible nasal endoscopy with simulated snoring was performed in those patients. The patients were asked to snore with an open mouth. The obtained data were documented as previously reported [16]. Briefly, the patients were examined by transnasal, fiberoptical examination and the structures of the upper airway were observed while the patients were asked to snore with an open mouth. The dorsal movement of the tongue base was graded from I° to IV° according to the visibility of the valleculae. The collapse of the pharynx was observed at velum and tongue base level and was graded from I° to V°. Data were documented as shown in Fig. 1. The examination was conducted under local anesthesia with 2% lidocaine applied endonasal in an upright, 45° and supine position.
Documentation and classification of anatomical changes during simulated snoring. a The dorsal movement during simulated snoring is classified (I°–IV°) according to the visibility of the valleculae. b The pharyngeal collapse of the pharynx at the tongue base level is graded according to the lateral or circular collapse during simulated snoring (I°–V°). c The pharyngeal collapse of the pharynx at velum level is graded according to the lateral or circular collapse during simulated snoring (I°–V°).
Sound recording
Snoring sounds during simulated snoring were digitally recorded. The examination and recording were performed in a quiet room. The examination under simulated snoring and the recording of snoring sounds were performed in an upright, 45° and supine position. Snoring was recorded by a microphone (ECM-2000, Monacor, Bremen, Germany), positioned 30 cm in front of the patient’s head. During nighttime polysomnography, the microphone was placed 30 cm above the patient’s head. The frequency response of the microphone was 30–20,000 Hz. Data were collected by a computer software (Cool Edit 2000, Syntrillium Software Corporation, Phoenix, AZ, USA) at a sampling rate of 44,100 Hz. The digitalized snoring sounds were analyzed by fast fourier transformation (FFT) on 16,384 samples using a Hanning window. The same parameters were used for the sound recording during simulated snoring and nighttime polysomngraphy.
Frequency analysis
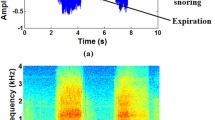
The obtained FFT frequency spectra were analyzed in terms of intensity peaks (IP). By applying FFT, a frequency response curve of a specific noise is obtained. The most dominant frequency of a noise is identified by its peak within the frequency response curve. Less prominent frequencies create lower peaks within the response curve. The first to fifth intensity peaks were documented for each single snoring event. Figure 2 demonstrates two examples of frequency analysis in a snoring episode with predominately low intensity peaks (Fig. 2a) and high intensity peaks (Fig. 2b). Depending on the intensity, the peaks were numbered from 1 to 5.
Examples of single nocturnal snoring events of rhythmic (a) and non-rhythmic (b) snoring. The intensity peaks are marked (1–5) in order, according to their intensity. Rhythmic snoring reveals low frequency peaks predominately below 800 Hz (a). Non-rhythmic snoring reveals high frequency peaks up to 3,500 Hz (b). The freqencies of each intensity peak are used for further calculations.
Intensity peaks of five consecutive snoring events were averaged and the average intensity peaks were analyzed with respect to nighttime snoring and simulated snoring (upright, 45°, supine position). For nighttime snoring, the snoring events were classified as rhythmic and non-rhythmic snoring. If the patients revealed a period of rhythmic snoring for at least 3 min, five consecutive snoring events were evaluated and these patients were classified as rhythmic snorers. If at least five obstructive apneas were present within a period of 3 min, the first snoring event after termination of each apnea was evaluated. In addition to the non-rhythmic snoring events, rhythmic periods of snoring were analyzed as well in these patients. These patients were classified as non-rhythmic snorers (n = 18). Rhythmic and non-rhythmic events of snoring were analyzed separately. For explanatory purpose, two examples of snoring episodes are displayed in Fig. 3.
Polysomnography
Data were obtained from 50 patients who underwent nighttime polysomnography. Cardio-respiratory and electro-encephalometric parameters were recorded by SIDAS GS polysomnograph (Heinen und Löwenstein, Bad Ems, Germany) using WIN NIGHT software for data acquisition and WIN DAY for data evaluation. Polysomnography and data analysis were conducted according to the guidelines of the American Sleep Disorders Association [1]. The following parameters were recorded: EEG, ECG, EOG, EMG, thoracal and abdominal respiratory excursion, blood oxygen saturation and body position. The apnea–hypopnea index (AHI), respiratory disturbance index (RDI), mean and minimal blood oxygen saturation (mean/min SAO2) and mean nocturnal heart rate were calculated automatically and manually revised from the raw data. Apneas were classified as periods of ten or more seconds without any oronasal airflow. They were named obstructive, central or mixed apneas according to their abdominal and/or thoracical effort. Hypopneas were classified as periods of ten or more seconds with an airflow reduction to 50% and blood oxygen desaturation by 3%. The AHI describes the respiratory events per hour.
Statistical analysis
Statistical analysis was conducted by SPSS software program (SPSS Inc., Chicago, Ill) performing a multifactorial analysis of variances/Spearman`s correlation and Wilcoxon rank sum test. A probability level of 5% was considered as significant.
Results
Patients’ baseline characteristics
A total of 50 male patients were included in the study. The mean age was 50 years (standard deviation (SD) 11.2; minimum: 31; maximum: 70). The mean body weight was 92 kg (SD: 17.2; minimum: 65; maximum: 138). The mean BMI was 30.1 (SD: 6.2; minimum: 23.8; maximum: 48.9).
Polysomnographic results
The mean AHI was 11 (SD 15; minimum: 0,1; maximum: 76,7). A total of 18 patients revealed an AHI ≥ 10, 42 ≤ 10 and 27 ≤ 5. The mean nocturnal blood oxygen saturation (SpO2) was 93% (SD: 2.9; minimum: 80; maximum: 97). The minimal nocturnal blood oxygen saturation was 77.5% (SD: 10.9; minimum: 51; maximum: 90). The mean nocturnal heart rate was 61.6 beats per minute (SD 9.2; minimum: 43; maximum: 92).
Descriptive analysis of results during simulated snoring
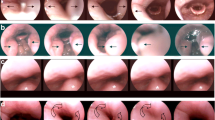
During simulated snoring under conditions awake, the uvula and velum were observed fluttering in all patients. Figure 4a shows an example of a snoring sequence at velum level with a fluttering uvula. This particular patient revealed an AHI of 0.5. In some patients, a dorsal movement of the tongue base could be observed. Figure 4b demonstrates a snoring sequence at tongue base level with a dorsal movement of the tongue base. The cranial edge of the epiglottis is covered by the tongue base during snoring. This particular patient revealed an AHI of 38.6.
Video sequence of simulated snoring of two exemplary patients. Sequence at velum level (a) of a patient with an AHI of 0.5. The uvula (+) is fluttering while snoring and contacts the dorsal pharyngeal wall (°). Sequence at tongue base level (b) of a patient with an AHI of 38.6. The tongue base (#) is shifted dorsal while simulated snoring, covering the epiglottis “*” and contacting lateral parts of the dorsal pharyngeal wall (°).
Correlation between results of simulated snoring in upright position and AHI
Dorsal movement of the tongue base
An increasing dorsal movement of the tongue base correlates with an increase in AHI (P = 0.001, r = 0.49) (Fig. 5a).
Correlation between the results of simulated snoring in upright position and AHI. The dorsal movement of the tongue base correlates with an increase in AHI (a). The collapse at the tongue base level correlates with an increase in AHI (b). There is no correlation between a collapse at the velum level and AHI (c). (n = 50)
Pharyngeal collapse at tongue base level
An increase in the pharyngeal collapse at tongue base level correlates with an increase in AHI (P = 0.028, r = 0.33) (Fig. 5b).
Pharyngeal collapse at velum level
An increase in the pharyngeal collapse at velum level does not correlate with an increase in AHI (P = 0.082, r = 0.28) (Fig. 5c).
Correlation between results of simulated snoring in the upright position and frequency analysis
Rhythmic snoring episodes were evaluated in all patients and are displayed in graph 6A, 7A and 8A (n = 50). If non-rhythmic episodes occured in these patients, the non-rhythmic episodes terinating an obstructive apnea were plotted in graph 6B, 7B and 8B (n = 18 of 50).
Dorsal movement of the tongue base
The first intensity peak frequency increases correlating with an increase of the dorsal movement of the tongue base in nocturnal rhythmic (P = 0.026, r = 0.47) and non-rhythmic snoring (P < 0.001, r = 0.52) (Fig. 6).
Correlation between the dorsal movement of the tongue base during simulated snoring and first intensity peak in rhythmic and non-rhythmic snoring events. The dorsal movement of the tongue base during simulated snoring correlates with an increase in frequency of the first intensity peak of nocturnal rhythmic (a) as well as non-rhythmic (b) snoring. (n = 50)
Pharyngeal collapse at tongue base level
There is no correlation between a pharyngeal collapse at tongue base level and an increase in the first intensity peak frequency in nocturnal rhythmic (P = 0.835, r = 0.07) and non-rhythmic snoring (P = 0.124, r = 0.24) (Fig. 7).
Correlation between the pharyngeal collapse at the tongue base level during simulated snoring and first intensity peak in rhythmic and non-rhythmic snoring events. There is no correlation between an increasing pharyngeal collapse and the first intensity peak in nocturnal rhythmic (a) and non-rhythmic (b) snoring. (n = 50)
Pharyngeal collapse at velum level
There is no correlation between a pharyngeal collapse at velum level and an increase in the first intensity peak frequency in nocturnal rhythmic (P = 0.634, r = 0.09) and non-rhythmic snoring (P = 0.065, r = 0.31) (Fig. 8).
Intensity peaks during simulated snoring
The intensity peaks 1–5 during simulated snoring revealed no differences between the different body positions (upright, 45°, supine, n = 50). The 25/75 percentiles of the first and second intensity peaks were located below 200 Hz as well as the percentiles of the third intensity peaks in upright and 45° position. The percentile of the third intensity peak in supine position increased up to 1,000 Hz. The percentiles of the fourth and fifth intensity peaks revealed a range of up to 3,000 Hz (Fig. 9a, b, c).
Frequency graphic of the intensity peaks 1–5 of simulated snoring in upright (a) 45 (b) and supine (c) position. The intensity peaks 1 and 2 range at a low frequency below 200 Hz. Peaks 3–5 increase up to a frequency of 3,000 Hz. There is no difference between the different positions at which simulated snoring is conducted. “Open square“ = mean value, percentile 25/50/75%, “x” = 1 and 99% value, “-” = minimum and maximum. (n = 50)
Intensity peaks of nocturnal rhythmic snoring
The percentile of the first intensity peak in rhythmic snorers (n = 32) ranged from 100 to 400 Hz. An increase in frequency of the following intensity peaks was detected (Fig. 10a).
Frequency graphic of the intensity peaks 1–5 for rhythmic (n = 32) (a) and non-rhythmic (n = 18) (b) nocturnal snoring events. Intensity peaks for rhythmic snoring events range from low frequencies in peaks 1 and 2 to high frequencies in peaks 3–5. The first intensity peak in non-rhythmic snoring events is located between 1,000 and 3,000 Hz. All consecutive peaks follow at high frequencies. There is a difference between peaks 1, 2, 3 and 5 between rhythmic and non-rhythmic nocturnal snoring. “Open square“ = mean value, percentile 25/50/75%, “x” = 1 and 99% value, “-” = minimum and maximum.
Intensity peaks of nocturnal non-rhythmic snoring
The percentile of the first intensity peak in non-rhythmic snorers (n = 18) ranged from 1,000 to 2,000 Hz. The percentile of the following intensity peaks remained at a high frequency between 400 and 2,000 Hz (Fig. 10b).
Differences between nocturnal snoring and simulated snoring
Nocturnal non-rhythmic snoring differs from nocturnal rhythmic and simulated snoring in IP 1–3. A three-dimensional plot of the different intensity peaks with mean and median values is shown in Fig. 11. Nocturnal rhythmic snoring does not differ from simulated snoring in all IP. The IPs of nocturnal non-rhythmic snoring reveal a significant higher frequency pattern than the IPs of rhythmic nocturnal and simulated snoring. The P values of nocturnal rhythmic and non-rhythmic snoring as well as simulated snoring at different positions are shown in Table 1.
Three-dimensional plot of mean (a) and median (b) values of snoring events under simulated and nocturnal conditions in relation to intensity peak and frequency. Non-rhythmic nocturnal snoring events (n = 18) show higher frequencies compared to rhythmic nocturnal (n = 32) snoring and simulated snoring (n = 50). Different positions of simulated snoring do not differ from each other and resemble nocturnal rhythmic snoring patterns. Significant differences are marked with asterisks. The corresponding P values are displayed in Table 1
Discussion
The pathogenesis of snoring sounds is still an unsolved task in somnology. The surgical treatment of snoring by different techniques is diagnostically based on: the medical history reported either by the patient himself or the partner, the clinical examination [25], and the polysomnographical or polygraphical examination including a snoring sound detection with integrated microphones or flow pressure measurement. The obtained data might give information about snoring in terms of intensity and quantity, but no further information about the quality of snoring sounds. Based on this diagnostic procedure, the site of obstruction for apneas as well as the pathogenesis of snoring sounds is rarely located exactly [2, 11, 16]. Accordingly, a potential surgical therapy of snoring does not necessarily treat the correct anatomical structures. The relatively high failure and recurrence rates after uvulo-palato-pharyngoplasty might be based on this problem [1, 12, 15, 17, 18].
Several more specific means of examination have been introduced to improve the diagnostic procedure in patients with snoring and suspected sleep disordered breathing. The method of choice seems to be the natural sleep endoscopy. The vibrating structures of the upper airways are directly visible under unaltered conditions. Unfortunately, this method does not serve as a routine diagnostic procedure because of limitations like relatively high logistical efforts and a potential lack of compliance of the patients. Drug-induced sleep endoscopy as an alternative means of inspecting the upper airway during sleep is frequently propagated as a reliable way to predict the site of obstruction along the upper airway [7, 23, 26]. But, studies have provided evidence that the drug-induced sleep does not resemble natural sleep and the information obtained from drug-induced endoscopy needs to be interpreted carefully [5, 10]. Moreover, despite the easy to obtain data of vibrating and obstructing structures of the upper airway by drug-induced sleep endoscopy, the snoring sounds obtained during this examination do not resemble natural snoring sounds completely [2]. These observations of Agrawal et al. raise the question if these differences in sound frequencies of drug-induced snoring and natural snoring are based on the effect of the used narcotic drug itself or if different vibrating structures are involved in the pathogenesis of snoring under natural nocturnal and drug-induced conditions. However, there is still a persisting uncertainty about drug-induced snoring in terms of evaluating the pathogenesis of snoring.
Another diagnostic method to predict the extent of sleep disordered breathing by simulated snoring was described previously [16]. The patients are examined by flexible nasal endoscopy under wake conditions and asked to simulate oral snoring. A correlation between the dorsal movement of the tongue base as well as the pharyngeal collapse at the tongue base level and the AHI was detected. These data show a reliable correlation between anatomical changes of the upper airway during simulated snoring and nocturnal obstructive events. As a consequence of these initial results, it seemed to be reasonable to compare the sound frequencies of simulated and nocturnal snoring to get more information about the pathogenesis of snoring. The upper airway is visible during simulated snoring and vibrating structures as pathogenetic structures of snoring are easy to detect. Despite the fact that this examination is conducted under conditions awake, the same structures might play a role in the creation of nocturnal snoring sounds. The aim of this study was to compare simulated and natural snoring sounds and correlate the data of the analyzed snoring events with the anatomical situation and polysomnographic data in order to attribute snoring sounds to a specific anatomical structure.
According to the presented data, there is a significant difference between nocturnal rhythmic and non-rhythmic snoring. Whereas rhythmic snoring reveals intensity peaks predominantly at lower frequencies, non-rhythmic snoring has a high frequency character throughout all intensity peaks. As the single events of non-rhythmic snoring were recorded after an obstructive apnea, it seems to be most likely that the mechanism of snoring differs from rhythmic snoring without obstructive events. Taking into account that the dorsal movement of the tongue base during simulated snoring correlates with an increase of the AHI as well as an increase in frequency of the first intensity peak in nocturnal snoring, it seems likely that the tongue base is involved in the mechanism of obstructing the upper airway. A forced respiratory effort during an obstructive apnea leading to an explosive opening sound contributes to a high frequency snoring sound. An observed dorsal movement of the tongue base and pharyngeal collapse at the tongue base level during simulated snoring correlates with an increase in the AHI. This observation seems to indicate a tendency for a nocturnal obstruction. The objection that the pure tongue base cannot be simulated by untrained patients needs to be considered. The patients are able to simulate easily velar/uvular snoring. Nevertheless, a dorsal movement of the tongue base during simulated snoring can be observed even under conditions awake when snoring at velar/uvular level. It should be emphasized that even this marginal dorsal movement of the tongue base and a tendency of pharyngeal collapse at the tongue base level, awake, correlate with nocturnal obstructive events. But apparently, this marginal dorsal movement of the tongue base or pharyngeal collapse at tongue base level, awake, is not distinct enough to create snoring sounds of high frequency due to a potential persisting muscular tone under conditions awake.
Contrary to non-rhythmic snoring events, the analyzed rhythmic nocturnal snoring events resemble simulated snoring sounds according to their intensity peaks. Most probably, those snoring sounds were produced at a different location from non-rhythmic snoring sounds. The position of the patient during simulated snoring does not play a major role. These snoring sounds could be created at the velum level, as the velum and uvula are observed to be fluttering during simulated snoring. From the presented data, it can be concluded that nocturnal rhythmic snoring is created at the same location as simulated snoring. This information might be useful for further surgical procedures.
Of course, additional research should focus on the exact documentation and movement of the oropharynx, velum and uvula during simulated or natural snoring to select potential patients for surgical treatments and to evaluate the post-surgical outcome.
A potential limitation of the presented study should be mentioned. The examination during simulated snoring under awake conditions was conducted under local anesthesia with 2% lidocaine endonasally applied. Several authors reported alterations of the nocturnal respiratory situation by topical applied anesthetics [4, 6, 9, 20, 27]. Nocturnal obstructive events are increased due to a blocked upper airway reflex mechanism. It is questionable if this effect is also present under awake conditions. The present study does not aim to simulate obstructions of the upper airway, but to simulate snoring, which might be associated with obstructions or occurs without obstructions as primary snoring. Apart from that, the produced snoring sounds are created by forced inspiration resulting in passive fluttering of the velum and uvula with a still persisting muscular tone of the upper airway due to the awake condition. The examination might certainly be conducted without local anesthesia with a well compliant patient accepting the slight inconvenience of nasal endoscopy. But it should be kept in mind that potential gagging, which is induced by the endoscope without local anesthesia could alter the results more than the local anesthesia itself.
Conclusion
The examination of the upper airway under simulated snoring is an easy to conduct method to examine a patient with a history of snoring. Frequency analysis of the obtained snoring sounds during simulated snoring reveals frequency patterns, which are in conformity with nocturnal frequency patterns of rhythmic snoring. Those snoring sounds are likely to be created at the velum level. Non-rhythmic snoring sounds differ from rhythmic nocturnal and simulated snoring sounds and are likely to be created at the tongue base level due to its positive correlation to nocturnal respiratory events. Different sites of snoring can be determined by frequency analysis of natural and simulated snoring sounds. The examination under simulated snoring might contribute to a more precise determination of the pathogenesis of snoring and might improve the outcome of surgery in snoring patients.
References
AARC (1995) AARC-APT (American Association of Respiratory Care-Association of Polysomnography Technologists) clinical practice guideline. Polysomnogra Respir Care 40:1336–1343
Agrawal S, Stone P, McGuinness K, Morris J, Camilleri AE (2002) Sound frequency analysis and the site of snoring in natural and induced sleep. Clin Otolaryngol Allied Sci 27:162–166
Beck R, Odeh M, Oliven A, Gavriely N (1995) The acoustic properties of snores. Eur Respir J 8:2120–2128
Berry RB, Kouchi KG, Bower JL, Light RW (1995) Effect of upper airway anesthesia on obstructive sleep apnea. Am J Respir Crit Care Med 151:1857–1861
Bonora M, St John WM, Bledsoe TA (1985) Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis 131:41–45
Chadwick GA, Crowley P, Fitzgerald MX, O’Regan RG, McNicholas WT (1991) Obstructive sleep apnea following topical oropharyngeal anesthesia in loud snorers. Am Rev Respir Dis 143:810–813
Croft CB, Pringle M (1991) Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci 16:504–509
Dalmasso F, Benedetto G, Pogolotti R, Righini G, Spagnolo R (1990) Digital processing of snoring sounds. Eur Respir J Suppl 11:528s–532 s
Deegan PC, Mulloy E, McNicholas WT (1995) Topical oropharyngeal anesthesia in patients with obstructive sleep apnea. Am J Respir Crit Care Med 151:1108–1112
Dolly FR, Block AJ (1982) Effect of flurazepam on sleep-disordered breathing and nocturnal oxygen desaturation in asymptomatic subjects. Am J Med 73:239–243
Dreher A, de la Chaux R, Klemens C, Werner R, Baker F, Barthlen G, Rasp G (2005) Correlation between otorhinolaryngologic evaluation and severity of obstructive sleep apnea syndrome in snorers. Arch Otolaryngol Head Neck Surg 131:95–98
Fernandez Julian E, Esparcia Navarro M, Garcia Callejo FJ, Orts Alborch MH, Morant Ventura A, de la Fuente L, Sanchez F, Marco Algarra J (2002) Clinical and functional analysis of long-term results of uvulopalatopharyngoplasty. Acta Otorrinolaringol Esp 53:269–280
Fiz JA, Abad J, Jane R, Riera M, Mananas MA, Caminal P, Rodenstein D, Morera J (1996) Acoustic analysis of snoring sound in patients with simple snoring and obstructive sleep apnoea. Eur Respir J 9:2365–2370
Hara H, Murakami N, Miyauchi Y, Yamashita H (2006) Acoustic analysis of snoring sounds by a multidimensional voice program. Laryngoscope 116:379–381
Hassid S, Afrapoli AH, Decaestecker C, Choufani G (2002) UPPP for snoring: long-term results and patient satisfaction. Acta Otorhinolaryngol Belg 56:157–162
Herzog M, Metz T, Schmidt A, Bremert T, Venohr B, Hosemann W, Kaftan H (2006) The prognostic value of simulated snoring in awake patients with suspected sleep-disordered breathing: introduction of a new technique of examination. Sleep 29:1456–1462
Hessel NS, Vries N (2004) Increase of the apnoea-hypopnoea index after uvulopalatopharyngoplasty: analysis of failure. Clin Otolaryngol Allied Sci 29:682–685
Hicklin LA, Tostevin P, Dasan S (2000) Retrospective survey of long-term results and patient satisfaction with uvulopalatopharyngoplasty for snoring. J Laryngol Otol 114:675–681
Hill PD, Lee BW, Osborne JE, Osman EZ (1999) Palatal snoring identified by acoustic crest factor analysis. Physiol Meas 20:167–174
McNicholas WT, Coffey M, McDonnell T, O’Regan R, Fitzgerald MX (1987) Upper airway obstruction during sleep in normal subjects after selective topical oropharyngeal anesthesia. Am Rev Respir Dis 135:1316–1319
Miyazaki S, Itasaka Y, Ishikawa K, Togawa K (1998) Acoustic analysis of snoring and the site of airway obstruction in sleep related respiratory disorders. Acta Otolaryngol Suppl 537:47–51
Petrou-Amerikanou C, Belazi MA, Daskalopoulou E, Vlachoyiannis E, Daniilidou NV, Papanayiotou PC (2005) Oral findings in patients with obstructive sleep apnea syndrome. Quintessence Int 36:293–298
Quinn SJ, Daly N, Ellis PD (1995) Observation of the mechanism of snoring using sleep nasendoscopy. Clin Otolaryngol Allied Sci 20:360–364
Quinn SJ, Huang L, Ellis PD, Williams JE (1996) The differentiation of snoring mechanisms using sound analysis. Clin Otolaryngol Allied Sci 21:119–123
Rombaux P, Bertrand B, Boudewyns A, Deron P, Goffart Y, Hassid S, Leysen J, Liistro G, Marien S, Moerman M, Remacle M (2002) Standard ENT clinical evaluation of the sleep-disordered breathing patient; a consensus report. Acta Otorhinolaryngol Belg 56:127–137
Steinhart H, Kuhn-Lohmann J, Gewalt K, Constantinidis J, Mertzlufft F, Iro H (2000) Upper airway collapsibility in habitual snorers and sleep apneics: evaluation with drug-induced sleep endoscopy. Acta Otolaryngol 120:990–994
White DP, Cadieux RJ, Lombard RM, Bixler EO, Kales A, Zwillich CW (1985) The effects of nasal anesthesia on breathing during sleep. Am Rev Respir Dis 132:972–975
Zonato AI, Bittencourt LR, Martinho FL, Junior JF, Gregorio LC, Tufik S (2003) Association of systematic head and neck physical examination with severity of obstructive sleep apnea-hypopnea syndrome. Laryngoscope 113:973–980
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herzog, M., Schieb, E., Bremert, T. et al. Frequency analysis of snoring sounds during simulated and nocturnal snoring. Eur Arch Otorhinolaryngol 265, 1553–1562 (2008). https://doi.org/10.1007/s00405-008-0700-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-008-0700-2