Abstract
We sought to analyze the predictive value of anthropometric, clinical and epidemiological parameters in the identification of patients with suspected OSA, and their relationship with apnoea/hypopnoea respiratory events during sleep. We studied retrospectively 433 patients with OSA, 361 men (83.37%) and 72 women (16.63%), with an average age of ±47, standard deviation ±11.10 years (range 18–75 years). The study variables for all of the patients were age, sex, spirometry, neck circumference, body mass index (BMI), Epworth sleepiness scale, nasal examination, pharyngeal examination, collapsibility of the pharynx (Müller Manoeuvre), and apnoea-hypopnoea index (AHI). Age, neck circumference, BMI, Epworth sleepiness scale, pharyngeal examination and pharyngeal collapse were the significant variables. Of the patients, 78% were correctly classified, with a sensitivity of 74.6% and a specificity of 66.3%. We found a direct relationship between the variables analysed and AHI. Based on these results, we obtained the following algorithm to calculate the prediction of AHI for a new patient: AHI = −12.04 + 0.36 neck circumference +2.2286 pharyngeal collapses (MM) + 0.1761 Epworth + 0.0017 BMI × age + 1.1949 pharyngeal examinations. The ratio variance in the number of respiratory events explained by the model was 33% (r 2 = 0.33). The variables given in the algorithm are the best ones for predicting the number of respiratory events during sleep in patients studied for suspected OSA. The algorithm proposed may be a good screening method to the identification of patients with OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnoea (OSA) is characterized by recurrent episodes of obstruction of the upper airways during sleep and poses a serious problem of public health. It is generally associated with intensive snoring and hypoxemia. Apnoeas cause broken sleep and diurnal somnolence, one of the most prominent symptoms in OSA patients, which must be treated in all cases [36]. Studies have been limited due to the absence of uniform criteria in the definition of the syndrome, as well as the difficulty to make polysomnographic (PSG) studies on large population groups. However, clinical knowledge of the syndrome and the development of simpler screening techniques have allowed some studies to be made. A study of 1,171 men and 1,347 women made by Franceschi et al. [12] found that 26 (1.2%) presented OSA (more than 5 apnoea/h), highlighting the fact that 8 were men and 18 women. Lavie [23] studied a total of 1,502 men, made a polysomnography in 78 candidates and found that 11 (0.89%) presented more than 10 apnoea/h. Gislason et al. [13] studied 3,100 Swedish men and found that 166 were in risk of OSA, and considered that the prevalence of OSA was 1.3%. Schmidt-Novara et al. [33], in a study of 1,195 people over 40, considered that the prevalence of OSA (apnoea–hypopnoea/h = 10) was 2.3% for men and 1.1% for women. Young et al. [40] in a sample of 602 study patients with polysomnography established a real prevalence of 2% for women and 4% for men.
As we know, OSA is almost always associated with obesity, although in children it is usually found in adenoid and amygdale hypertrophy cases and, less frequently, linked to craniofacial anomalies [39]. In young adults, we can find it in severe obesities, and primarily in non-obese individuals due to craniofacial anomalies [10]. At the same time, obesity and body mass index (BMI), and the prevalence of chronic diseases that contribute towards OSA and aggravate its repercussions increase with age [3, 4]. By sexes, android obesity is characterized by more fat accumulation in the upper trunk and neck, and in groups with the same BMI, men have a larger cervical circumference than women. Thus, OSA appears earlier and is more severe in men than in women [8, 27]. BMI is the most widely accepted test to quantify obesity. It is calculated by dividing weight in kilograms by the square of height in meters (BMI = kg/m2). However, this index gives a global idea of obesity, whereas affectation of the cervical spaces (produced by narrowness of upper airway, causing snores and apnoeas) may vary widely. So, cervical circumference measurements show a better correlation with OSA that the BMI. In adult men, a neck size of more than 43 cm. predicts severe OSA and, in the case of men who snore and have a larger neck than that, the prevalence of OSA is 30% [7]. In women, a neck size of more than 38 cm. increases the risk of OSA [14]. At the same time, performing Epworth sleepiness scale (ESS) is very useful. However, by itself it is not a diagnostic test, although some authors believe there is a good correlation between high scores and sleep pathologies [19, 20]. Finally, the Müller Manoeuvre (forced inspiration through the nose and mouth covered) was added to the fiberscope examination by Sher et al. [35] in order to identify the degree of collapse or obstruction of the airway at the level of the soft palate and the nasopharyngeal and oropharyngeal union. So, studying other diagnostic approaches should be given high priority, given the proven relationship between OSA and a 2–7 increase in the probability of having a traffic accident [2], a probable increase cardiovascular morbimortality [18, 21, 24, 34] and the high effectiveness of the treatment with continuous positive pressure of the airway (CPAP) fundamentally in the most symptomatic forms [1]. Several studies have been used as a diagnostic tool, by using multivariate methods to construct prediction equations [11, 17, 22, 31]. The purpose of our study was to identify OSA on the basis of several epidemiological and clinical variables, including ENT observations. Our research sought to find alternatives to expensive procedures such as polysomnography, in order to develop a predictive model of OSA.
Materials and methods
We studied retrospectively a sample of 433 patients with OSA, 361 men (83.37%) and 72 women (16.63%), aged ±47 standard deviation of ±11.10 years (range, 18–75 years). Patients were taken over a period of 4 years and the study variables in all of the patients were: age (years), sex, spirometry, neck circumference (circumference of the neck measured in cm at level of the cricothyroid membrane), BMI, Epworth sleepiness scale, nasal examination (anterior rhynoscopy and rigid endoscopy), pharyngeal examination (pharyngoscopy), Müller Manoeuvre (collapsibility of the pharynx), and apnoea-hypopnoea index (AHI). By age, three subgroups were considered: <43, 43–52 and >52 years. Taking into account the importance of the BMI and age in OSA, these parameters were classified into several subgroups. Thus, the BMI was studied with two types of cuts: BMI1, where BMI ≤ 24 kg/m2 was normal and ≥25 kg/m2 overweight and obesity, and BMI2, where ≤24 kg/m2 was normal, 25–29 kg/m2 overweight and ≥30 kg/m2 obesity.
The degree of diurnal somnolence was determined by using the Epworth sleepiness scale, in which a patient answers a questionnaire on the possibility of remaining asleep in eight different situations. Patients score the possibility of remaining asleep in each one of the situations from 0 to 3 (0 would be null probability; 1 would be light probability; 2 moderate probability, and 3 high probability), where <10 is normal and >10 means diurnal hypersomnolence, and >16 occurs almost exclusively in patients with severe or moderate OSA, narcolepsy or idiopathic hypersomnolence [8, 27].
All patients were explored by nasofibroscopy (Olympus® nasofibroscope), using the Müller Manoeuvre. This manoeuvre consists of making a forced inspiration effort with mouth and nose closed during a flexible nasofibroscopy, to observe collapse at the velopharyngeal and hypopharyngeal level. Airway collapse at this level was observed by displacement of the base of the tongue and the sidewalls of the pharynx in a medial direction. The degree of collapse at each level was determined according to the scale established by Petri et al. [29], as follows: (a) minimum collapse, (b) reduction of the cross-sectional area to 50%, and (c) reduction to 75–100%. Based on this, a score of the collapse was established and patients were classified into two subgroups depending on whether their pharynx was normal or abnormal: (1) those who showed no collapse (collapse 0) and (2) those who displayed a certain degree of collapse (collapse 1–4).
A nocturnal polysomnography was performed in all of the patients using a 19-channel polysomnography system Alice 3®: 2 of EEG recording (CÁ2 and CÂ1), 2 of ocular movements, 1 of oronasal flow measured by a Healthdyne® thermistor (USA), 1 of snoring, 1 for the Allegiance® (Allegiance Healthcare Corporation, USA) nasal pressure cannula, connected to a PTAF2® (Pro-tech, USA) pressure transducer model, 1 chest band, 1 abdominal band, both of the piezoelectric type, 1 for submentonian musculature activity, 2 for leg movements, as well as continuous monitoring of ECG, O2 saturation, 1 channel for the altimeter and another one of body position. We considered OSA patients to be those who presented an index of apnoea-hypopnoea/hour ≥10 in the PSG. OSA was understood as >90% absence or reduction of the respiratory signal for more than 10 s in the presence of respiratory effort detected by the thoracic-abdominal bands; and hypopnoea, a discernible reduction (>30, <90%) in the amplitude of the respiratory signal of more than 10 s, with 3% desaturation.
Statistical analysis
A logistic regression analysis was used to predict the probability of having OSA from clinical and epidemiological variables. Multivariate logistic regression was used to determine the best model for distinguishing OSA patients from healthy ones, using a stepwise procedure. A ROC curve was plotted to evaluate the area under the curve of the combination of variables affecting AHI.
A simple linear regression was used to study the relationship between clinical and epidemiological parameters and apnoea/hypopnoea respiratory events during sleep. Stepwise multiple regressions were then made, with AHI as the dependent variable. Independent variables were entered into the equation: age, sex, BMI, BMI1 and BMI2, neck circumference, ESS, nasal examination, pharyngeal examination and pharynx collapsibility (Müller Manoeuvre). The AHI distribution showed statistically significant deviation from normality by the Shapiro–Wilks test. When AHI was the dependent variable in the multiple linear regression equation, a square root transformation was used to reduce the deviation from normality. Stepwise multiple linear regressions were used to identify variables that made an important contribution to the variability of apnoea/hypopnoea respiratory events during sleep.
All results were obtained using SAS Inc. Institute v8.02 and Stata8 software.
Results
Sensitivity and specificity of clinical and epidemiological parameters to identify OSA patients
Age (classified into three different groups or as a continuous variable), neck circumference, BMI, ESS (as a dichotomizing and continuous variable), pharyngeal examination and pharyngeal collapse (Müller Manoeuvre) were the significant variables. Considering the classification variables, the patients of the oldest group (>52) were 3.8 times more likely to have OSA that those of younger age group (<43), which was statistically significant. By BMI, depending on the cut-off point, it was found that in BMI1 the patients with BMI ≥ 25 had 2.5 times more possibilities of having OSA that those with a normal BMI (P = 0.002), and that in BMI2 when BMI ≥ 30, the possibility of having OSA was 3.3 times greater than in the patients with a normal BMI. Patients with diurnal hypersomnolence (ESS ≥ 10) had 2.5 times more possibilities of having OSA than those with a normal OSA test. When the pharyngeal examination presented some abnormality, the possibility of having OSA was (2.8 times when the pharynx was normal. Finally, patients who presented some degree of pharyngeal collapse in the Müller Manoeuvre had almost five times more possibilities of having OSA than those who showed no collapse. Table 1 shows that the risk of being an OSA patient increases with age (5.8%), neck circumference (17.5%), BMI (13%) and ESS (8%).
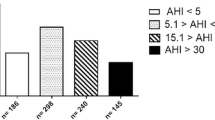
To obtain best possible explanatory model for predicting a diagnosis of patients with OSA (P < 0.05), we found that when age, neck circumference and ESS increase by 1 unit, the possibility of having OSA is 4.5, 9.5 and 7.3% times greater, respectively. As far as the Müller Manoeuvre is concerned, patients with pharyngeal collapse had 3.5 times more possibilities of having OSA than those who did not present collapse, Table 2. Using this model, we obtained a sensitivity of 74.6% and a specificity of 66.3%, Fig. 1. After making a ROC curve, we obtained an area under the curve of 0.78. This means that the probability that the model classified OSA patients correctly was 78% (Fig. 2).
A predictive model of the relationship between clinical and epidemiological parameters and respiratory events during sleep
We found a direct relationship between respiratory events and the analysed variables, with statistical meaning for all the variables, except in the ones for nasal examination and tobacco consumption (P = 0.07, 0.63, respectively). A new variable was introduced, BMI × age, because the initial model suggested an interaction between age and the BMI. Neck circumference was the variable that best explained the variance of the dependent variable (22.1%), followed, in decreasing order, by the BMI (14.79%), BMI × age interaction (14.49%), the pharyngeal collapse observed in the Müller Manoeuvre (12.41%) and, finally, the pharyngeal examination variable (10.90%), Table 3.
Table 4 gives the results of the multiple linear regressions with a selection of the most significant variables. Neck circumference, ESS, pharyngeal examination, Müller Manoeuvre and interaction between age and the BMI were selected in the final model, indicating a direct relationship between the dependent variables and the respiratory events. The ratio of the variance in the number of respiratory events explained by the model was 33% (r 2 = 0.33).
Based on these results, we obtained the following algorithm to calculate the prediction of AHI for a new patient:
-
AHI = −12.04 + 0.36 neck circumference + 2.2286 pharyngeal collapses (MM) + 0.1761 ESS + 0.017 BMI × age + 1.1949 pharyngeal examinations,
where pharyngeal collapse (MM) = 0 for Müller Manoeuvre without collapse and one for evidence of some degree of collapse, and pharyngeal examination = 0 for patients who did not present pharynx anomalies and one for evidence of pharyngeal pathology.
Discussion
Many studies [5, 6, 28, 32, 37] have evaluated the ability of questionnaires on sleep to predict OSA. Viner et al. studied the possibility of using medical histories and physical examinations for screening in 410 patients (220 OSA and 190 RS). They found a sensitivity of 94% and a specificity of 28% in OSA patient detection, where the parameters with greater statistical significance for distinguishing OSA from RS were age, BMI, sex (masculine) and snoring. The probability of identifying a patient with OSA correctly was 77%, and of obtaining an area under the curve ROC = 0.77, which is similar to ours. They obtained a low specificity in relation to the one obtained in our study’s model (66.3%). Haponik et al. [16] found that reports from the room companions of patients suspected of having OSA obtained a sensitivity of 64% and concluded that a single medical history and examination were insufficient as screening methods to identify patients with sleep apnoea. Nevertheless, these studies excluded from their analysis parameters of high relevance and statistical meaning in relation to OSA, such as age and the BMI. Hoffstein et al. [15] studied 594 patients and obtained statistical meaning to predict OSA with age, sex, BMI, reports from room companions and pharyngeal examination. They established a formula to predict the AHI in a new patient. Their model, based on the examiner’s subjective impression of the medical history and clinical examination, obtained a sensitivity of 60% and a specificity of 63% in the detection of patients with OSA. They conclude that clinical characteristics such as the medical history and a physical examination, can predict OSA in around 50% of patients suspected of suffering the disorder, which is not sufficient for reasonable OSA detection. Crocker et al. studied 100 patients with suspected sleep apnoea and found that the BMI, age, apnoeas observed by room companions and arterial hypertension had a significant correlation with their AHI. When they obtained a model to consider the probability of an AHI > 15 in 105 new patients, they observed a sensitivity of 92% and a specificity of 51%. They concluded that a model based on breathing to predict disorders during sleep, would be useful to screen patients with suspected OSA, and that the model could be used to reduce the need for a nocturnal PSG in nearly one third of the cases.
However, Scharf et al. [32] made a questionnaire on the sleep of 40 patients chosen for their clinical characteristics, including age and BMI, and did not find a significant correlation between those characteristics and the AHI, although they admitted that perhaps the sample was not sufficiently large to be able to prove the correlation. Flemons et al. [11] obtained a model in which they included a questionnaire, BMI, HTA and neck circumference, and obtained a sensitivity of 76% and a specificity of 54%. Maislin et al. [25] developed a prediction model that included a questionnaire, BMI, age and sex, with which they obtained sensitivity of 87% and specificity of 35%. Pradhan et al. [30] studied 150 patients and found that the most significant OSA prediction variables were age, sex, BMI and sonorous snoring. They concluded that a screening algorithm based on clinical data would have to reduce the number of polysomnographies by 8%, whereas the same model associated to a home pulsioximetry would reduce the amount by 13%.
Rowley et al. [31] made a prospective study in 370 patients of four previously published models [6, 22, 25, 38]. When applying the prediction models on their sample, they found that sensitivity, and mainly specificity, were lower than what the authors of previously published articles had obtained. Rowly et al. tried to explain these differences adducing that their population’s average BMI (37.1 kg/m2) was higher than that of the other authors. Furthermore, in their population, the sex distribution was more balanced in relation to the other studies (191 men and 179 women). They conclude that although these models were not able to distinguish the presence or absence of OSA, they would be useful for giving priority to a split night PSG (the same night is divided in two parts, one to obtain a diagnostic PSG and the other to establish the optimal pressure for treatment with CPAP) in those patients with a high probability of having OSA, according to these models. In conclusion, most authors, in accordance with our results, conclude that it is possible to identify OSA patients on the basis of medical history and the findings of the physical examination.
Predictive model
Several studies have tried to find diagnostic methods that allow differentiating patients with OSA before carrying out a polysomnographic study. Logistic regression models use various combinations of clinical variables to predict the presence of OSA for different AHI cut-off points in patients sent by doctors specialized in sleep [9, 15, 17, 22]. Results have varied, depending fundamentally on the existing medical probability of having OSA and on the AHI cut-off point, although generally they have presented a high sensitivity (greater than 85%) and a low specificity (less than 55%) [31].
Martinez et al. [26] have described a logistic equation that presented an excellent predictive capacity in patients with an AHI = 30. This equation included four habitual variables in the published prediction equations to date, such as the presence of HTA, the presence of observed and repeated apnoeas, and the ESS and BMI values, all of which are considered dichotomizing variables, the latter two with cut-off points of 11 and 30, respectively. According to odds ratio (OR) value reach for each one of these variables, it would seem that, using higher AHI cut-off points than usual, there was no a qualitative change in the variables that were finally included in the equation, although there did exist a significant change in the relative weight of the same ones regarding their predictive power. The presence of HTA (OR = 11.9) and a high score in ESS (OR = 4.47) was more important than age, sex, and the anthropometric presence of apnoeas or variables (neck circumference or the BMI). This study would not be applicable to those individuals whose companions do not know of the appearance of such apnoeic events, for instance, in individuals who live or sleep alone. The explanation of why the equation of these authors presented an important diagnostic and predictive capacity can be complex. Probably, this is due to the fact that this study presents two characteristics that make the calculated prediction equation more globally effective than the usual one: the high cut-off point chosen and the high probability pre-test for presenting it, since none of the variables that comprise of the final equation presents low sensitivity. Rather, each one is moderate (between 50 and 67%). Therefore, it is important to highlight that the diagnostic value of our equation could change if applied to populations with characteristics that differed from those of our population.
Conclusions
The results obtained in this study allow us to conclude that the variables shown in the algorithm obtained are those that best predict the number of respiratory events during sleep in patients studied for suspected OSA. Thus, it would be possible to base OSA patient identification on medical histories and the findings of physical examinations, because the algorithm proposed may be a good screening method to the identification of patients with OSA.
References
Ballester E, Badía JR, Hernández L, Carrasco E, de Pablo J, Fornas C, Rodriguez-Roisin R, Montserrat JM (1999) Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 159:495–501
Barbé F, Pericás J, Muñoz A, Findley L, Antó JM, Agustí AG (1998) Automobile accidents in patients with sleep apnea syndrome. An epidemiological and mechanistic study. Am J Respir Crit Care Med 158:18–22
Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R (1995) Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med 151:82–86
Chervin RD, Guilleminault C (1996) Obstructive sleep apnea and related disorders. Neurol Clin 14:583–609
Chervin RD, Aldrich MS, Pickett R, Guilleminault C (1997) Comparison of the results of the Epworth sleepiness scale and the multiple sleep latency test. J Psychosom Res 42:145–155
Crocker BD, Olson LG, Saunders NA, Hensley MJ, McKeon JL, Allen KM, Gyulay SG (1990) Estimation of the probability of disturbed breathing during sleep before a sleep study. Am Rev Respir Dis 142:14–18
Davies RJ, Ali NJ, Stradling JR (1992) Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnoea syndrome. Thorax 47:101–105
Dealberto MJ, Ferber C, Garma L, Lemoine P, Alperovitch A (1994) Factors related to sleep apnea syndrome in sleep clinic patients. Chest 105:1753–1758
Deegan PC, McNicholas WT (1996) Predictive value of clinical features for the obstructive sleep apnoea syndrome. Eur Respir J 9:117–124
Ferguson KA, Ono T, Lowe AA, Keenan SP, Fleetham JA (1995) The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest 108:375–381
Flemons WW, Whitelaw WA, Brant R, Remmers JE (1994) Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med 150:1279–1285
Franceschi M, Zamproni P, Crippa D, Smirne S (1982) Excessive daytime sleepiness: a 1-year study in an unselected inpatient population. Sleep 5:239–247
Gislason T, Almqvist M, Eriksson G, Taube A, Boman G (1988) Prevalence of sleep apnoea syndrome among Swedish men—an epidemiological study. J Clin Epidemiol 41:571–576
Guilleminault C, Stoohs R, Kim YD, Chervin R, Black J, Clerk A (1995) Upper airway sleep-disordered breathing in women. Ann Intern Med 1(122):493–501
Hoffstein V, Szalai JP (1993) Predictive value of clinical features in diagnosing obstructive sleep apnea. Sleep 16:118–122
Haponik EF, Smith PL, Meyers DA, Bleecker ER (1984) Evaluation of sleep-disordered breathing. Is polysomnography necessary? Am J Med 77:671–677
Harding SM (2001) Prediction formulae for sleep-disordered breathing. Curr Opin Pulm Med 7:381–385
He J, Kryger MH, Zorick FJ, Conway W, Roth T (1988) Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest 94:9–14
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Johns MW (1992) Reliability and factor analysis of the Epworth sleepiness scale. Sleep 15:376–381
Kiely JL, McNicholas WT (2000) Cardiovascular risk factors in patients with obstructive sleep apnoea syndrome. Eur Respir J 16:128–133
Kirby SD, Eng P, Danter W, George CF, Francovic T, Ruby RR, Ferguson KA (1999) Neural network prediction of obstructive sleep apnea from clinical criteria. Chest 116:409–415
Lavie P (2002) Incidence of sleep apnea in a presumably healthy working population: a significant relationship with excessive daytime sleepiness. Sleep 25:312–318
Leung RS, Bradley TD (2001) Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med 164:2147–2165
Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, Schwab RJ, Dinges DF (1995) A survey screen for prediction of apnea. Sleep 18:158–166
Martinez Garcia MA, Soler Cataluña JJ, Roman Sanchez P, Cabero Salt L, Gimenez Ibañez I, Gastaldo Palop T (2003) Clinical predictors of sleep apnea-hypopnea syndrome susceptible to treatment with continuous positive airway pressure. Arch Bronconeumol 39:449–454
Millman RP, Carlisle CC, McGarvey ST, Eveloff SE, Levinson PD (1995) Body fat distribution and sleep apnea severity in women. Chest 107:362–366
Olson LG, King MT, Hensley MJ, Saunders N (1995) A community study of snoring and sleep-disordered breathing. Prevalence. Am J Respir Crit Care Med 152:711–716
Petri N, Suadicani P, Wildschiodtz G, Bjorn-Jorgensen J (1994) Predictive value of Muller maneuver, cephalometry and clinical features for the outcome of uvulopalatopharyngoplasty. Evaluation of predictive factors using discriminant analysis in 30 sleep apnea patients. Acta Otolaryngol 114:565–571
Pradhan PS, Gliklich RE, Winkelman J (1996) Screening for obstructive sleep apnea in patients presenting for snoring surgery. Laryngoscope 106:1393–1397
Rowley JA, Aboussouan LS, Badr MS (2000) The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep 1(23):929–938
Scharf SM, Garshick E, Brown R, Tishler PV, Tosteson T, McCarley R (1990) Screening for subclinical sleep-disordered breathing. Sleep 13:344–353
Schmidt-Nowara WW, Coultas DB, Wiggins C, Skipper BE, Samet JM (1990) Snoring in a Hispanic-American population. Risk factors and association with hypertension and other morbidity. Arch Intern Med 150:597–601
Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O’Connor GT, Boland LL, Schwartz JE, Samet JM (2001) Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med 163:19–25
Sher AE, Thorpy MJ, Shprintzen RJ, Spielman AJ, Burack B, McGregor PA (1985) Predictive value of Muller maneuver in selection of patients for uvulopalatopharyngoplasty. Laryngoscope 95:1483–1487
Stradling JR (1995) Sleep-related breathing disorders. Obstructive sleep apnoea: definitions, epidemiology, and natural history. Thorax 50:683–689
Stradling JR, Crosby JH (1991) Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax 46:85–90
Viner S, Szalai JP, Hoffstein V (1991) Are history and physical examination a good screening test for sleep apnea? Ann Intern Med 1(115):356–359
Ward SL, Marcus CL (1996) Obstructive sleep apnea in infants and young children. J Clin Neurophysiol 13:198–207
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santaolalla Montoya, F., Iriondo Bedialauneta, J.R., Aguirre Larracoechea, U. et al. The predictive value of clinical and epidemiological parameters in the identification of patients with obstructive sleep apnoea (OSA): a clinical prediction algorithm in the evaluation of OSA. Eur Arch Otorhinolaryngol 264, 637–643 (2007). https://doi.org/10.1007/s00405-006-0241-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-006-0241-5