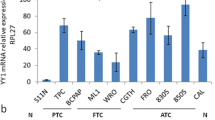
Abstract
We investigated the expression of COX-1 and COX-2 in normal thyroid tissue, follicular adenoma and well-differentiated thyroid carcinomas and evaluated the difference in COX-1 and COX-2 expression. Ten normal thyroid tissues, ten follicular adenomas, ten papillary carcinomas and ten follicular carcinomas were analyzed by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) for expression of COX-1 and COX-2 mRNA. In addition, immunohistochemical staining was performed to find the expression of the two enzymes in normal thyroid tissues and thyroid neoplasia. Expression of COX-1 mRNA in the normal thyroid tissues, follicular adenomas and both well-differentiated carcinomas was similar and weak. However, COX-2 mRNA was strongly expressed in the well-differentiated carcinomas compared to those of normal thyroid tissue and follicular adenoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer represents just 1~2% of all malignancies and 5~24% of thyroid nodules [4]. Most patients with thyroid cancer are treated with total thyroidectomy and hormone replacement therapy for the rest of their lives. Patients with thyroid nodules that could not be diagnosed by non-invasive techniques or by fine-needle cytology usually receive surgery in order to differentiate benign from malignant disease. The identification of specific molecular markers to assist in discriminating potentially malignant from benign thyroid tumors and demonstration of its role could be helpful in searching for a molecular target for the prevention and treatment of thyroid cancers. Several molecular markers have been suggested to be associated with thyroid cancers. However, there is little consensus about their value in the differentiation of benign and malignant thyroid nodules.
Recently, multiple studies have highlighted the relevance of COX-2 in head and neck cancers as well as other epithelial carcinomas. It is important to investigate the role of COX-2 in the carcinogenesis of thyroid carcinoma for finding out the target material in its prevention and treatment. It also contributes to the rationale of using a selective COX-2 inhibitor as an adjuvant modality of treatment. However, the association of COX-2 with the development of thyroid carcinoma has not yet been clarified. Furthermore, a report that COX-1 is not an innate constituent, but an inducing substance in the vascular endothelium [5, 7] gives impetus to the necessity of an investigation concerning the origin of COX-1 in the thyroid gland, a highly vascular organ. Here, we investigate the localization of COX-1 and COX-2 protein and the difference in the expression of the COX-1 and COX-2 gene in the normal thyroid gland, follicular adenoma and well-differentiated thyroid carcinomas.
Materials and methods
Tissue samples
Tissues were obtained from surgical biopsy procedures performed at the Department of Otolaryngology and Head and Neck Surgery, Korea University College of Medicine. Thyroid tissues were recovered from thyroidectomy: ten patients with thyroid follicular adenoma, ten patients with thyroid papillary carcinoma (there was no follicular type) and ten patients with follicular carcinoma. As a control, the most remote normal thyroid tissues were obtained in the surgical specimen from ten patients with nodular hyperplasia. One portion was immediately flash frozen in liquid nitrogen and stored at −70°C for subsequent RNA studies. Another portion was fixed with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH7.4), stored overnight at 4°C and then embedded in paraffin for immunohistochemical staining. The tissue procurement procedures were approved by the institutional review board at Korea University.
Extraction of RNA
Tissues were homogenized in 1 ml of Trizol reagent (Gibco BRL, Tucson, Ariz.), and RNA was extracted according to the manufacturer’s instructions. Samples were air-dried and resuspended in water treated with diethyl pyrocarbonate and were kept on ice for immediate use or stored at −70°C. Aliquots of RNA were treated with RQ1 RNAase-free Dnase (Promega, Madison, Wis.) according to the manufacturer’s instructions. Concentrations of RNA were determined spectrophotometrically, and the integrity was checked by electrophoresis in agarose gels containing formaldehyde.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
The total RNA from each sample was reverse-transcribed in 20 μl of reaction mixture containing 2.5 U of Moloney murine leukemia virus reverse transcriptase (Gibco, BRL) and 50 pmol of random hexanucleotides (Pharmacia, UK) at 42°C for 60 min. Based on the published sequences, oligonucleotide primers were synthesized commercially at Bioneer Co. (Daejon, South Korea) for PCR as follows: 5’-TGC CCA GCT CCT GGC CCG CCG CTT-3’ and 5’-CCA TGG CCC AAG GCC TTG-3’ for COX-1 and 5’-TTC AAA TGA GAT TGT GGG AAA ATT GCT-3’ and 5’-AGA TCA TCT CTG CCT GAG TAT CTT-3’ for COX-2 and 5’-CCA CCC ATG GCA AAT TCC ATG GCA C-3’ and 5’-TCT AGA CGG CAG GTC AGG TCC ACC-3’ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Amplification of the complementary DNA was carried out using 35 cycles at 94°C for 45 s, 55°C for 30 s and 72°C for 1 min followed by a final extension cycle of 72°C for 7 min. The specificity of the 304(COX-1) and 322(COX-2)-base pair (bp) PCR product was verified by the predicted size, restriction digestion and DNA sequencing. To establish the specificity of responses, negative controls were used in which input RNA was omitted or in which RNA was used, but reverse transcriptase was omitted. To ensure RNA quality, all preparations were subjected to the analysis of GAPDH expression. To analyze semiquantitatively the result of RT-PCR, we scanned the gel images and measured the intensity of the PCR product through the use of NIH Image software (National Institutes of Health, Bethesda, Md.). We determined the relative intensity of individual bands on a gel image as the ratio of the intensity of COX-1 and COX-2 to the intensity of GAPDH.
Immunohistochemical staining for COX protein
The paraffin blocks were sliced into 5-μm-thick sections. Deparaffinizations with xylene and rehydration with 100% and then 75% alcohol were done serially. Paraffin sections from the paraformaldehyde-fixed thyroid tissues were treated with 3% hydrogen peroxide methanol to block endogenous peroxidase and were incubated with a mouse monoclonal antibody to COX-1 and COX-2 (Cayman Chemical, Ann Arbor, Mich.) used at a dilution of 1:100 and incubated overnight at 4°C in a humidified chamber. Immunoreactive COX-1 and COX-2 were visualized with a Vectastatin Elite ABC Kit (Vector Lab, Inc, Burlingame, Calif.). Controls included the substitution of primary or secondary antibody with phosphate-buffered saline.
A pathologist who did not know the result of RT-PCR counted positive cells for COX-1 and COX-2 by monitoring at least 1,000 cells in at least five randomly selected fields. A positive cell was defined when the immunoreactivity was clearly observed in their cytoplasm. The percentages of immunopositive cells were classified into three categories: diffusely positive (2), >50% of cells were positive; heterogeneneously positive (1), 10~49% of cells were positive; negative (0), <10% of the cells were positive. The immunointensity was classified into four groups as follows: 0, negative; 1, weak; 2, moderate; 3, strong. Positive controls were set as 3. The immunoractivity scores for each group were calculated by multiplication of the values for the two variables.
Statistical analysis
Data were provided using mean ± SD. The Kruskal-Wallis test was used to compare median values among four groups within each of expression. Multiple comparisons with a 5% level of significance were also performed using Miller’s (1981) method. All statistical analyses, except multiple comparisons, were conducted using SAS statistical software version 9.1 (SAS Institute, Cary, N.C.). The reported P -values were two-tailed, and P <0.05 was considered statistically significant.
Results
Reverse transcriptase-polymerase chain reaction
The RT-PCR studies showed that thyroid tissue contained mRNA encoding for COX-1 and COX-2. The PCR products from the thyroid tissue had the sizes (304 bp for COX-1 and 322 bp for COX-2) that were expected from the selected primers. COX-1 mRNA was weakly expressed in the normal thyroid gland, follicular adenoma, papillary carcinoma and follicular carcinoma (Fig. 1). The data of the COX-1/GAPDH mRNA ratio and COX-2/GAPDH mRNA ratio for each group are described in Table 1. There were no significant differences in the density of the COX-1 bands among them (Fig. 2A). COX-2 mRNA was also expressed in the normal thyroid gland, follicular adenoma, papillary carcinoma and follicular carcinoma (Fig. 1). The density of the bands representing COX-2 in papillary carcinoma and follicular carcinoma was significantly increased compared with those in the normal or follicular adenoma (Fig. 2B). There were no significant differences in the density of both COX-1 and COX-2 bands between the normal thyroid tissue and follicular adenoma and between the papillary carcinoma and follicular carcinoma (Fig. 2A, B).
Expression of COX-1 and COX-2 mRNA in human thyroid adenomas and well-differentiated carcinomas by reverse transcriptase-polymerase chain reaction (RT-PCR). Ethidium bromide-stained agarose gel showing the presence of 304 bp and 322 bp RT-PCR product using specific primers for COX-1 and COX-2; PC papillary carcinoma; FC follicular carcinoma
Comparison of the COX-1/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ( A) and the COX-2/GAPDH mRNA ratio ( B). Each ratio of normal, follicular adenomas, papillary carcinomas ( pc) and follicular carcinomas ( fc) was similar and not significantly different in COX-1. however; in COX-2, it is significantly increased in the papillary and follicular carcinomas compared with normal and follicular adenomas. There are no significant differences between the normal and follicular adenomas and between the papillary and follicular carcinomas
Immunohistochemical findings
Immunohistochemical study showed that the expressions of COX-1 and COX-2 were localized to the perinuclear cytoplasm in the normal thyroid tissue, follicular adenoma, papillary carcinoma and follicular carcinoma. The immunoreactivity scores of COX-1 in the normal thyroid tissue (Fig. 3A) and follicular adenoma (Fig. 3B) were 1 for all. The scores for papillary carcinoma (Fig. 3C) and follicular carcinoma (Fig. 3D) were all 2. A higher immunoreactivity score for COX-2 was obtained in both papillary carcinoma (Fig. 3G) and follicular carcinoma (Fig. 3H), which was 6 for each, compared with those in the normal thyroid tissue, 1 (Fig. 3E) or the follicular adenoma, 2 (Fig. 3F).
The expression of COX-1 ( A – D) and COX-2 ( E – H) in the human thyroid tissue. Immunohistochemical staining using monoclonal antibody against COX-1 and COX-2 reveals immunoreactivity for COX-1 and COX-2 in the thyroid follicular epithelia of the normal gland ( A), follicular adenoma ( B), papillary ( C) and follicular carcinoma ( D). The immunoreactivities of COX-1 are similarly weak. However, COX-2 immunoreactivity was strong in papillary ( G) and follicular carcinoma ( H) compared to the normal gland ( E) and follicular adenoma ( F). The differences of immunoreactivity between normal gland and follicular adenoma and between papillary and follicular carcinoma are not significant (ABC method, original magnification ×400)
Discussion
Cyclooxygenase (COX), also known as prostaglandin-endoperoxide H synthase, is the first oxidase in the process of prostaglandin (PG) production from arachidonic acid (AA). This enzyme has both fatty acid COX activity for the synthesis of PGs from AA and PG hydroperoxidase activity for the synthesis of PGH2 from PGG2 [9]. Two isoforms of COX have been characterized: COX-1 and COX-2. They share over 60% identities at the amino acid level and have similar enzymatic activities, but although they catalyze the same reaction, these isoforms may have distinct biological functions [11]. COX-1 acts to maintain cellular homeostasis. It is found at high levels of abundance in most tissues and cells in culture. The levels of COX-1 expression are relatively invariant with respect to tissue involvement in disease or cytokine and mitogen activity. It is the enzymatic activity attributable to COX-1 that is currently thought to contribute predominantly to basal PGE2 production in healthy tissue and to maintain the integrity of renal and enteric epithelium [12]. On the contrary, COX-2 is an inducible enzyme that is ordinarily not detected in most tissues under normal conditions. However, it is induced in a variety of tissue by growth factors, oncogenes, inflammatory stimuli and tumor promoters [13]. Therefore, the pathophysiological role of COX-2 has been connected to inflammation, reproduction and carcinogenesis. Recent studies have highlighted the relevance of COX-2 in the development of numerous types of epithelial cancers. An elevated expression of COX-2 has been reported in the squamous cell carcinomas of the head and neck, colon, stomach, breast, esophagus, lung, liver and pancreas. Prostaglandins, the products of COX-2 activity, have been implicated in carcinogenesis by promoting angiogenesis, inhibiting apoptosis, increasing malignant cell invasion and stimulating cell proliferation [12, 15]. However, the exact role of COX-2 in the progression of cancer is not well understood. There is also cumulative evidence that selective COX-2 inhibitors prevent carcinogenesis in experimental animals and that these compounds induce apoptosis and inhibit growth in several types of cancer cells [7, 11].
The human thyroid is a common site for the occurrence of inflammatory and neoplastic disease. The currently undefined role of COX-2 in the mediation of thyroid disease is of potential importance. Several recent articles have been reported on cyclooxygenase, mainly COX-2, expression in human thyroid neoplasms. Commonly, they reported that COX-2 was expressed in papillary carcinoma, follicular carcinoma, [2, 3, 6, 8, 10] and thyroiditis [3, 8, 10]. The level of expression in these well-differentiated carcinomas was higher than that of benign tumors, which may provide insight into the role of COX-2 in the progression from benign to malignant thyroid tumors [2, 6, 10]. And no or little expression was observed in the normal and poorly differentiated carcinoma [3, 8, 10]. Only one report investigated COX-1 and COX-2 activity simultaneously and yet just in medullary thyroid carcinoma [1]. Our study demonstrated that the COX-1 protein and gene were expressed in the normal thyroid tissue, follicular adenoma, papillary carcinoma and follicular carcinoma with a similar degree of expression. This result was consistent with that of another study that was conducted using a different human organ [18]. The COX-2 protein and gene were also expressed in all four groups of our specimens. Their expressions were elevated in papillary and follicular thyroid carcinomas compared with the expressions in normal thyroid tissue and follicular adenoma. The difference between normal and follicular adenoma and between papillary carcinoma and follicular carcinoma was not remarkable and not significant in our study. In our study, the normal thyroid tissue turned out to have both COX-1 and COX-2 protein and gene even though the levels were low; these results are opposed to the results of other studies. There is one report that suggested higher COX-2 expression in papillary carcinoma than in follicular carcinoma, which was different from our result and suggested that COX-2 may contribute predominantly to the progression of papillary carcinoma [6].
One study described that COX-2 expression is constitutive [4], and another study reported that COX-1 expression is inducible [9, 15]. There is a lack of consistent results concerning the origin of COX-1 and COX-2 in the thyroid gland, a highly vascular endocrine organ. In our study, we could localize the COX-1 and COX-2 proteins in the cytoplasm and the vascular cores of human thyroid follicular epithelial cells. In addition, the difference in the expression of COX-1 and COX-2 mRNA among the normal thyroid tissue, follicular adenoma and well-differentiated carcinoma could be demonstrated. These findings imply that there may be a possible role for COX-1 in the cytoplasm of the thyroid follicular cells and that COX-2 may be a trigger in the neoplastic progression to well-differentiated carcinoma in the human thyroid gland. One mechanism for the elevated tumor associated with COX-2 expression could be related to the marked repression of the transcription of the COX-2 gene by wild-type p53. Loss of the wild-type p53 would then be associated with the increased expression of COX-2 mRNA and protein [14].
The results of our study provide another rationale for the usefulness of selective COX-2 inhibitors. Thyroid cancer differs from other epithelial cell carcinomas in the head and neck region because it shows hormone dependence. In conclusion, the expression of COX-1 in the normal thyroid follicular epithelium and the tumor cells suggested that COX-1 could have a constant role in human thyroid epithelial cells. And over-expression of COX-2 in papillary and follicular carcinoma implied that COX-2 might play a key role in the carcinogenesis of the human thyroid gland. However, further studies about other inflammatory conditions or poorly differentiated carcinomas are needed in order to define the exact action mechanism and role of COX-1 and COX-2 in cancer development in the human thyroid gland.
References
Bell CD, Vidal S, Kovacs K, Horvath E, Rotondo F (2002) An immunohistochemical survey of nine cases of medullary carcinoma of thyroid including reactivity for Cox-1 and Cox-2 enzymes. Endocr Pathol 13:331–340
Casey MB, Zhang S, Jin L, Kajita S, Lloyd RV (2004) Expression of cyclooxygenase-2 and thromboxane synthase in non-neoplastic and neoplastic thyroid lesions. Endocr Pathol 15:107–116
Cornetta AJ, Russell JP, Cunnane M, Keane WM, Rothstein JL (2002) Cyclooxygenase-2 expression in human thyroid carcinoma and Hashimoto’s thyroiditis. Laryngoscope 112:238–242
Gianoukakis AG, Cao HJ, Jennings TA, Smith TJ (2001) Prostaglandin endoperoxide H synthase expression in human thyroid epithelial cells. Am J Physiol Cell Physiol 280:701–708
Hla T (1996) Molecular characterization of the 5.2 KB isoform of the human cyclooxygenase-1 transcript. Prostaglandins 51:81–85
Ito Y, Yoshida H, Nakano K, Takamura Y, Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Matsuura N, Kuma K, Miyauchi A (2003) Cyclooxygenase-2 expression in thyroid neoplasms. Histopathol 42:492–497
Kawamori T, Rao CV, Seibert K, Reddy BS (1998) Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res 58:409–412
Kim SJ, Lee JH, Yoon JS, Mok JO, Kim YJ, Park HK, Kim CH, Byun DW, Suh KI, Yoo MH (2003) Immunohistochemical expression of COX-2 in thyroid nodules. Korean J Intern Med 18:225–229
Narko K, Ristimaki A, MacPhee M, Smith E, Haudenschild CC, Hla T (1997) Tumorigenic transformation of immortalized ECV endothelial cells by cyclooxygenase-1 overexpression. J Biol Chem 272:21455–21460
Nose F, Ichikawa T, Fujiwara M, Okayasu I (2002) Up-regulation of cyclooxygenase-2 expression in lymphocytic thyroiditis and thyroid tumors: significant correlation with inducible nitric oxide synthase. Am J Clin Pathol 117:546–551
Okajima E, Denda A, Ozono S, Takahama M, Akai H, Sasaki Y, Kitayama W, Wakabayashi K, Konishi Y (1998) Chemopreventive effects of nimesulide, a selective cyclooxygenase-2 inhibitor, on the development of rat urinary bladder carcinomas initiated by N -butyl- N -(4-hydroxybutyl)nitrosamine. Cancer Res 58:3028–3031
Sheng H, Shao J, Morrow JD, Beauchamp RD, Dubois RN (1998) Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 58:362–366
Smith WL, Garavito RM, DeWitt DL (1996) Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and −2. J Biol Chem 271:33157–33160
Subbaramaiah K, Altorki N, Chung WJ, Mestre JR, Sampat A, Dannenberg AJ (1999) Inhibition of cyclooxygenase-2 gene expression by p53. J Biol Chem 274:10911–10915
Tsujii M, Kawano S, Tsujii S, Sawaoka H, Hori M, DuBois RN (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93:705–716
Vane JR, Bakhle YS, Botting RM (1998) Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120
Wang C, Crapo LM (1997) The epidemiology of thyroid disease and implications for screening. Endocrinol Clin North Am 26:189–218
Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, Yoshimura N, Hla T, Wada S (2000) Expression of cyclooxygenase-2 in prostate carcinoma. Cancer 89:589–596
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HM., Baek, SK., Kwon, SY. et al. Cyclooxygenase 1 and 2 expressions in the human thyroid gland. Eur Arch Otorhinolaryngol 263, 199–204 (2006). https://doi.org/10.1007/s00405-005-0999-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-005-0999-x