Abstract
The aim of this study was to determine the prevalence of human papillomaviruses (HPV) types 6, 11, 16, 18, 31, 33, 42, 51, 52, 56 and 58 in laryngeal squamous cell carcinoma specimens using immunohistochemical reactions and to correlate the presence of HPV with the clinical and pathological characteristics of these patients. Tissue samples were collected from 40 patients with primary laryngeal squamous cell carcinoma (LSCC) and from 33 subjects with non-neoplastic laryngeal lesions or laryngeal nodules, which served as a control group. Human papilloma virus was detected in 6 (15%) of the 40 patients. Five (83.4%) of six patients with HPV positive tumors had G2 (moderately differentiated), one patient (16.6%) had G3 (poorly differentiated), and no patient with HPV positive tumor had a G1 (well-differentiated) tumor. Four (66.6%) of the six HPV positive tumors were in the supraglottic region, one (16.6%) tumor was located in the glottis, and one (16.6%) HPV positive tumor was in the subglotic region. Five (83.4%) of six HPV positive tumors were T3-T4, and one was T2. Three of six HPV positive patients had no clinically evident cervical lymph nodes (N0), and three of the HPV positive patients were N1 or N2. Human papillomavirus was not detected in any of the samples from the control group. The presence of HPV infection in 15% of the cases may suggest a possible role in the etiology of laryngeal squamous cell carcinoma. However, no significant correlation between HPV incidence and histological grading and clinical staging could be demonstrated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillomaviruses cause hyperproliferative cutaneous and/or mucosal epithelial lesions in higher vertebrates, including humans [20]. Mucosal epitheliotropic human papillomaviruses (HPV) have been associated with a variety of lesions, including benign anogenital warts, premalignant intraepithelial neoplasms and invasive cancers, particularly of the uterine cervix [9, 23]. Human papillomaviruses are small double-stranded DNA viruses and are responsible for pathological conditions ranging from benign skin warts to invasive cervical carcinomas [10]. Papillomaviruses are members of the papovaviridae family. They are non-enveloped icosahedral DNA viruses, which infect humans as well as a variety of animals. The papillomavirus virion capsid consists of two proteins, L1 and L2. L1 is the major capsid protein and when expressed in eukaryotic expression systems is able to self-assemble into virus-like particles (VLPs) [8, 19]. The expression of L1 protein results in the self-assembly of virus-like particles, which have the size, shape and conformational epitopes of virion capsids. L1 is able to self-assemble into VLPs, morphologically and immunologically very similar to virions, which are both necessary and sufficient for binding to the cell surface [8, 18]. Eighty-five genotypes have been fully characterized; approximately 120 additional isolates represent only partially characterized putative novel genotypes [24].
The larynx is the most common site of malignancy in the upper aerodigestive tract. The tumor is a squamous cell carcinoma (SCC) in over 90% of the cases, and the main predisposing factors are smoking and alcohol use. Viral infections, nutritional deficiency and local dietary customs have also been implicated in the etiology of laryngeal cancer [11, 12, 14]. The aim of this study was to determine the prevalence of HPV types 6, 11, 16, 18, 31, 33, 42, 51, 52, 56 and 58 in laryngeal squamous cell carcinoma specimens using immunohistochemical reactions and to correlate the presence of HPV with the epidemiological and clinicopathological characteristics of these patients.
Material and methods
Tissue samples were collected from 40 patients with primary laryngeal squamous cell carcinoma (LSCC) during diagnostic microlaryngoscopy. The group consisted of 34 males and 6 females, aged between 40 and 74 years (mean 57.5 years) treated in our center from 1999–2001. The group of consecutive patients was operated on by the first author. The patients had not undergone previous radiotherapy or chemotherapy. A control group consisted of 33 patients, 19 males and 14 females, aged between 21 and 71 years (mean 41.4 years), with non-neoplastic laryngeal lesions or laryngeal nodules.
The clinicopathological features of the patients and 3-year follow-up results are shown in Table 1. Tumors were staged according to TNM classification (UICC, 1992) and graded as well (G1), moderately (G2) and poorly (G3) differentiated. The specimens were collected during a diagnostic microlaryngoscopy and biopsy for histopathological diagnosis.
Paraffin-embedded tissues were serially sectioned at 4-µm thickness. After de-paraffinization, sections were stained for HPV monoclonal mouse antibodies and heated in a water bath with DAKO Target Retrieval Solution for 40 min. After washing with TBS, it was incubated with mouse monoclonal anti-human papillomavirus antibodies Clone K1H8, DAKO (types 6, 11, 16, 18, 31, 33, 42, 51, 52, 56 and 58) in dilution 1:50 with DAKO antibody diluents for 60 min at room temperature. The samples were than incubated for 30 min with DAKO LASB system at room temperature. Visualization of the reaction was acquired with DAB solution. After that, staining with Mayer’s hematoxylin was performed. DAKO positive control slides were used as a positive control. In the negative control, original antibodies (DAKO) were not added. The antigens detected in the nuclei of proliferating squamous cell carcinoma were regarded as a positive reaction. The presence of HPV in cancer samples, in relation to location, tumor size, lymph-node involvement and histopathological grade, was analysed using the χ2 test.
Survival rates at the 3-year follow-up were calculated using Kaplan-Meier curves. The relationship between HPV presence and survival rate was calculated using the F Cox test for small groups
Results
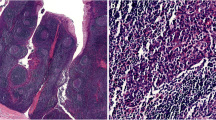
Human papillomavirus was detected in 6 (15%) of the 40 patients with LSCC (Fig. 1A and B), and it was not detected in any of the samples from the control group. Clinical and pathological features of HPV positive patients are shown in Table 1. Five males and one female were HPV positive. Five (83.4%) of six samples with HPV-positive tumors were G2, one sample (16.6%) was G3 and no sample with HPV-positive tumor was G1 (well differentiated). Four (66.6%) of the six HPV positive tumors were supraglottic, one (16.6%) was located in the glottis and one in the subglottic region. Five (83.4%) of the six HPV positive tumors were T3 or T4, and one was T2. Three of six HPV positive patients had no clinically evident cervical lymph nodes (N0), and three HPV positive patients were N1 or N2. All but one of the HPV positive patients were heavy cigarette smokers, and three of six frequently consumed alcohol.
Eighteen (45%) of 40 patients with LSCC lived at least 3 years after the initial treatment. Twenty-one (52.5%) patients died because of the disease and one patient because of a cardio-respiratory event. In 23 patients from the whole group of 40 patients, local or regional recurrence or distant metastasis occurred. Five (83.3%) of six patients who were HPV positive lived at last 3 years, and one (16.3%) patient died 9 months after surgery because of local recurrence. One HPV-positive patient was alive at the 3-year follow-up with successful treatment of regional recurrence. Thirteen (39.4%) of 18 HPV negative patients were alive 3 or more years after treatment.
No significant correlation was found between HPV incidence and the histological grade and clinical stage of the tumor. The difference in survival rates between the groups of HPV positive versus HPV negative patients was also not significant.
Discussion
Recently, several techniques have been used to detect the presence of HPV in tissues, for example, Southern-blot analysis, which was previously considered to be the most sensitive assay or in situ hybridization analysis that could be performed on paraffin-embedded sections. Those techniques have the disadvantage of requiring a sizable quantity of nucleic acids and take several days to perform. PCR is more sensitive than other techniques, but its high sensitivity may lead to product carryover or DNA contamination, which can create problems in diagnostic applications. Gomez et al. [5] showed that in situ hybridization is a more sensitive technique than immunohistochemistry for confirming the presence of HPV in severe dysplasias and carcinomas in situ of the uterine cervix. Furthermore, in situ hybridization provides much more information than immunohistochemistry since it permits the identification of the HPV types causing the lesion.
Immunochisochemistry can be performed on a small specimen. It is possible to use the diagnostic tissue sample also to identify the presence of HPV infection. Immunohistochemical staining using the monoclonal antibody K1H8 appears to recognize a non-conformational internal linear epitope. Only fibrillary structure, but not capsid structure, is observed in the negative staining. This epitope would be common among different types of HPV. On the other hand, monoclonal antibodies raised by using intact virions as the immunogen recognize a type-specific epitope of HPV-1. Such specific epitopes are considered to be present only on the surface of the capsid [22]. The antigen in the cytoplasm of some koilocytes is consistent with the results of in situ DNA hybridization [3].
Garcia-Miliàn et al. [4] demonstrated amplification of consensus primers for the HPV L1 gene using PCR technique in 6 (18.1%) of 33 laryngeal squamous cell carcinoma. When these samples were amplified using primers specific for the HPV 16 and 18 E7 gene, ten new cases were found. This increased the former data up to 16 (48.5%) in HPV DNA positivity. In this study we detected six (15%) patients with positive reaction with monoclonal antibody K1H8 of HPV. Previously, we [13] had determined the presence of the HPV DNA in laryngeal SCC assessed by polymerase chain reaction using specific primers for HPV-16 and 18 E6/E7. There were seven (35%) HPV-positive specimens. Five (25%) specimens were HPV-16 positive, and four (20%) specimens were HPV-18 positive. In two cases both HPV-16 and HPV-18 were present.
Almadori et al. [1] detected HPV DNA in 9 of 45 (20%) patients using the PCR technique. They also showed that the HPV positive tumors were 55% G2 and 33% G3. Fifty-five percent of the HPV positives had no clinical cervical lymph nodes (N0), and 78% of the HPV positive cancer patients were T3-T4.
Almadori [1] described more HPV-positive patients with supraglottic tumors than with tumors in other locations. Hoshikawa et al. [6] detected HPV DNA in 17.6% of laryngeal cancer patients by PCR. Glottic laryngeal carcinoma was HPV positive in 44.4% of the cases, and it was significantly higher than 8.7% HPV positive supraglottic tumors. Previous studies paid no attention to the precise primary site of laryngeal carcinoma. HPV infection may be more frequent in the vocal cords than in other laryngeal sites. Our data showed that the HPV positive tumors were more frequent in the supraglottic than in glottic regions, but patients with supraglottic tumors were the majority of the whole group as well.
Some authors claim that HPV DNA is more frequently detected in well-differentiated [2, 7] than in poorly differentiated squamous cell carcinoma of the upper aerodigestive tract [16]. In our study, HPV was detected more often in moderately (83.4%) and poorly (16.6%) differentiated than in well-differentiated tumors. Still, the differences were not statistically significant.
The presence of HPV infection in the premalignant and hyperplastic laryngeal lesions is controversial. Poljak et al. [17] analyzed the prevalence of HPV in laryngeal epithelial hyperplastic lesions using PCR and in situ hybridization methods. The HPV was present in only 2 of 88 specimens, and the authors suggested that most of hyperplastic lesions in the larynx are not associated with HPV infection.
Nishiaka et al. [15], using the PCR method, demonstrated the presence of HPV 16/18 in 5 of 27 (18.5%) patients with laryngeal cancer and in 2 of 33 (6%) of non-cancer control cases. They suggested that the presence of HPV is one of the risk factors of SCC.
Smith et al. [21] detected HPV in 25% (11/44) of patients with laryngeal cancer, in 30% (3/10) of patients with laryngeal leukoplakia and in 16.7% (2/12) of non-cancer controls, which did not include patients with laryngeal nodules. In the present study the HPV infection was not detected in laryngeal nodules, which served as a negative control.
Conclusion
The presence of HPV infection in 15% of the cases may suggest a possible role of HPV in the etiology of laryngeal squamous cell carcinoma. The immunohistochemical staining can be performed for L1 HPV detection on paraffin-embedded sections collected during diagnostic procedures.
References
Almadori G, Cadoni G, Cattani P, Posteraro P, Scarano E, Ottaviani F, Paludetti G, Maurizi M (1996) Detection of human papillomavirus DNA in laryngeal squamous cell carcinoma by polymerase chain reaction. Eur J Cancer 32A:783–788
Anwar K, Nakakuki K, Naiki H, Inuzuka M (1993) Ras gene mutations and HPV infection are common in human laryngeal carcinoma. Int J Cancer 2:22–28
Burns J, Graham AK, Frank C, Fleming KA, Evans MF, McGee JO (1987) Detection of low copy human papilloma virus DNA and mRNA in routine paraffin sections of cervix by non-isotopic in situ hybridisation. J Clin Pathol 40:858–864
Garcia-Milian R, Hernandez H, Panade L, Rodrriguez C, Gonzalez N, Valenzuela C, Arana MD, Perea SE (1998) Detection and typing of human papillomavirus in benign and malignant tumors of laryngeal epithelium. Acta Otolaryngol 118:754–758
Gomez F, Abad MM, Munoz E, Alonso MJ, Roldan M, Najera ML, Cermeno F, Paz JI, Bullon A, Lopez-Bravo A (1992) Study of infection by human papillomavirus in severe dysplasias and carcinomas in situ of the uterine cervix using immunohistochemistry and in situ hybridization. Eur J Histochem 36:137–142
Hoshikawa T, Nakajima T, Uhara H, Gotoh M, Shimosato Y, Tsutsumi K, Ono I, Ebihara S (1990) Detection of human papillomavirus DNA in laryngeal squamous cell carcinoma by polymerase chain reaction. Laryngoscope 100:647–650
Ishibashi T, Matsushima S, Tsunokawa Y, Asai M, Nomura Y, Sugimura T, Terada M (1990) Human papillomavirus DNA in squamous cell carcinoma of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg 116:294–298
Kinrbauer R, Booy F, Cheng N, Lowy R, Schiller JT (1992) Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 89:12180–12184
Lorincz AT, Reid R, Jenson AB, Greenberg MD, Lancaster W, Kurman RJ (1992) Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol 79:328–337
Lowy DR, Howley PM (2001) Papillomaviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (eds) Field’s virology, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 2231–2264
Maier H, Dietz A, Gewelka U, Heller WD, Weidauer H (1992) Tobacco and alcohol and the risk of head and neck cancer. Clin Invest 70:320–327
Millon RR, Cassisi NJ, Clark JR (1989) Cancer of the head and neck. In: DeVita VT, Hellman S, Rosenberg SA (eds) Cancer: principles and practice of oncology. JB Lippincott, Philadelphia, pp 488–590
Morshed K, Stenzel A, Szymański M, Różyńska K, Siwiec H, Gołąbek W, Wojcierowski J (2001) Detection of human papillomavirus type 16 and 18 in laryngeal cancer using PCR. Otolaryng Pol 55:29–33
Muscat JE, Wynder EL (1992) Tobacco, alkcohol, asbestos, and occupational risk factors for laryngeal cancer. Cancer 69:2244–2251
Nishioka S, Fukushima K, Nishizaki K, Gunduz M, Tominaga S, Fukazawa M, Monden N, Watanabe S, Masuda Y, Ogura H (1999) Human papillomavirus as a risk factor for head and neck cancers—a case-control study. Acta Otolaryngol [Suppl] 540:77–80
Perez-Ayala M, Ruiz-Cabello F, Esteban F, Concha A, Redondo M, Oliva MR, Cabrera T, Garrido F (1990) Presence of HPV 16 sequences in laryngeal carcinomas. Int J Cancer 46:8–11
Poljak M, Gale N, Kambic V (1997) Human papillomaviruses: a study of their prevalence in the epithelial hyperplastic lesions of the larynx. Acta Otolaryngol [Suppl] 527:66–69
Roden RBS, Kirnbauer R, Jenson AB, Lowy DR, Schiller JT (1994) Interaction of papillomaviruses with the cell surface. J Virol 68:7260–7266
Rose RC, Bonnez W, Reichman RC, Garcea RL (1993) Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of virus-like particles. J Virol 67:1936–1944
Shah KV, Howley PM (1996) Papillomaviruses. In: Fields BN, Knipe DM, Howley PM (eds) Virology, 3rd edn, vol 2. Lippincott-Raven, Philadelphia, pp 2077–2110
Smith EM, Kurt FS, Jeffrey A, Hoffman HT, McCulloch T, Turek LP, Haugen TH (2000). Human papillomavirus and the risk of laryngeal cancer. Ann Otol Rhinol Laryngol 109:1069–1076
Yaegashi N, Jenison SA, Valentine JM, Dunn M, Taichman LB, Baker DA, Galloway DA. Yaegashi N, Jenison SA, Valentine JM, Dunn M, Taichman LB, Baker DA, Galloway DA (1991) Characterization of murine polyclonal antisera and monoclonal antibodies generated against intact and denatured human papillomavirus type 1 virions. J Virol 65:1578–1583
Zur Hausen H (1994) Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol 186:131
Zur Hausen H (1999) Papillomaviruses in human cancers. Proc Assoc Am Physicians 111:581–587
Acknowledgements
The project was supported by a grant from the Polish State Committee for Scientific Research (no. 3 P05C 06224).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morshed, K., Korobowicz, E., Szymański, M. et al. Immunohistochemical demonstration of multiple HPV types in laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 262, 917–920 (2005). https://doi.org/10.1007/s00405-005-0925-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-005-0925-2