Abstract
Purpose
Kisspeptins regulate the trophoblast invasion. The disturbance of this process might lead to the development of preeclampsia (PE). Diabetes mellitus (DM) is associated with the high rate of this complication. The main hypothesis was to investigate the placental protein expression of kisspeptin-1 (KISS1) and its receptor (KISS1R) in diabetic, preeclamptic, and healthy pregnancies.
Methods
Placentae (n = 65) were divided into the following groups: the control group (n = 20), either PE or non-PE type-1 diabetes mellitus (T1DM) (n = 10), either PE or non-PE type-2 diabetes mellitus (T2DM) (n = 10), either PE or non-PE gestational diabetes mellitus (GDM) (n = 10) and preeclampsia without diabetes (PE) (n = 15). Immunohistochemistry analysis was used for demonstrating the presence and location of KISS1/KISS1R in placental tissue and to measure the area of immunopositive expression. Correlation analyses were performed to detect the links between protein expression of these biomarkers and the main obstetric outcomes.
Results
The highest placental protein expressions of KISS1 were detected in the PE (35.4%) and GDM (33.2%) groups. In case of DM, levels of KISS1 expression depended on the presence of PE and were higher compared with DM no PE and control groups: (30.6%) in T1DM + PE and (30.1%) in T2DM + PE group. The lowest expression was detected in the control group (14.1%). The expression of KISS1R was higher in DM and PE compared to the control group. We detected the strong direct link between PE and placental expression of KISS1 (r = 0.81) and KISS1R (r = 0.56), and inverse correlation link between KISS1 and preterm birth weight (r = − 0.73). The low correlation links were found between KISS1 and IUGR (r = 0.29), and preterm birth (r = 0.24). The same trend was detected for KISS1R. We did not find any significant correlations between placental expressions of KISS/KISS1R and placental weight or HbA1c levels.
Conclusion
Increased expression levels of KISS1 and KISS1R in case of diabetes mellitus may play a role in the altered placentation process and lead to the development of preeclampsia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Syncytiotrophoblast invasion into the uterine spiral arteries is one of the most crucial moments for the developing placenta and fetus. An adequate invasion provides an appropriate blood flow, nutrient and oxygen supply for the growing fetus, but a dysregulation associated with a wide spectrum of pregnancy complications [1]. This mechanism is regulated by cytokines, hormones, and growth factors that are produced by both the maternal and fetal tissues. The family of kisspeptins (metastins) may be considered as one of the key regulators of this mechanism [1, 2].
Kisspeptins regulate the implantation and placental development [1, 3]. The trophoblast is one of the sources for the synthesis of kisspeptins [1]. Additionally, extra placental structures synthesize these peptides [4]. The key role among all the kisspeptins in the gestational process is played by kisspeptin-1 (KISS1). Kisspeptin-1 acts via a G protein-coupled receptor (GPR-54) and activates KISS1R in trophoblast cells [5]. There are several KISS1 mechanisms responsible for the regulation of placental invasion. Ohtaki et al. (2001) showed that the KISS1 phosphorylated focal adhesion kinase and paxillin, which are required for cell migration [5]. Another mechanism of decreasing cell migration for KISS1 is to diminish the matrix metalloproteinase-9 (MMP9) expressions by reduced NFkB (NFKB1) [6]. Therefore, kisspeptin-1 and its receptor might play an important role in the control of trophoblast invasion and subsequent placental development [3].
The expressions of KISS1 and KISS1R have been demonstrated in a variety of human tissues including the hypothalamus, aorta, coronary artery, and umbilical vein [4]. However, the highest expression of these proteins was observed in the placenta [3, 4]. The mRNA and protein placental expression of KISS1 and KISS1R are the highest in the first trimester of pregnancy and decrease to the end of term [7]. Therefore, the activity of these peptides is associated with the most critical period of invasion regulation. The expression of KISS1 is highly observed in syncytiotrophoblast cells with localization in villous and extravillous cytotrophoblast cells [8]. Serum circulation levels of KISS1 in pregnancy increase dramatically to the end of term and fall rapidly after delivery [9]. These observations confirmed the role of KISS1 and KISS1R in early pregnancy as regulators for placental development [8]. Altered placental expression of KISS1 and KISS1R may be associated with poor placentation and linked with the development of adverse perinatal outcomes such as fetal intra-uterine growth restriction (IUGR), preterm birth, and preeclampsia (PE) [7].
PE is one of the most severe pregnancy complications, which affects up to 8% of the women [10]. The precise pathophysiological mechanisms underlying this disorder remain unclear. It is suggested that the inadequate remodeling of uterine spiral arteries during the invasion of trophoblasts leads to the failure of transformation processes into low-resistance vessels [10]. As a result, these vessels are capable of reacting to vasoactive triggers. It may induce local ischemia and endothelial dysfunction in the placental–uterine interface [11]. Ultimately, it leads to the development of hypertension and fetal hypoxia, which are typical for PE. Risk factors for PE include increased maternal age, family history, black race, obesity, parity, pre-existing, and gestational diabetes mellitus (GDM) [10].
Diabetes mellitus (DM) is one of the most unfavorable diseases during pregnancy, which confidentially leads to the development of adverse perinatal outcomes [11]. It is known that the DM is associated with structural and functional placental disturbances including impairment of cellular function, extracellular matrix, and basement membrane [12]. The severity of these changes depends on the type of diabetes (pre-gestational type 1, pre-gestational type 2 or GDM) and glycemic control [13]. Despite many reports referred to the placental expression of KISS1 and KISS1R in case of PE, there is a lack of these trials in diabetic pregnancy. Understanding the expression of factors involved in trophoblast invasion across the maternal–fetal interface is critical in providing further insights about the pregnancy-related pathologies associated with DM.
The objective of the study was to investigate the placental protein expression of KISS1 and KISS1R in women with different types of DM (1, 2, and GDM), PE and normal pregnancy, and match the results with the main obstetrical outcomes.
Materials and methods
The study was carried out as a retrospective cohort study. The participants were recruited from D.O. Ott Research Institute of Obstetrics, Gynaecology and Reproductive Medicine, Saint Petersburg, Russia. The inclusion criteria for this study were: singleton pregnancy, women with type-1 or -2 diabetes mellitus, gestational diabetes, and an informed consent to take part in the study. We excluded cases of DM with nephropathy, diabetes insipidus, twin pregnancy, severe extragenital pathology, and those who did not give an informed consent. The study was approved by the local ethical committee.
The women (n = 65) were divided into the following groups and subgroups:
- 1.
Control group; n = 20
- 2.
Group T1DM; n = 10
T1DM + PE; n = 5
T1DM no PE; n = 5
- 3.
Group T2DM; n = 10
T2DM + PE; n = 5
T2DM no PE; n = 5
- 4.
Group GDM; n = 10
GDM + PE; n = 5
GDM no PE; n = 5
- 5.
Group PE no DM; n = 15
Body mass index (BMI) was calculated for each participant. The diagnosis of GDM was set up according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) thresholds: fasting plasma glucose ≥ 5.1 mmol/l (92 mg/dl); 1-h plasma glucose ≥ 10.0 mmol/l (180 mg/dl); 2-h plasma glucose ≥ 8.5 mmol/l (153 mg/dl) [14], and PE according to the definition of the International Society for the Study of Hypertension in Pregnancy (ISSHP) [15]. The correlation analyses have been performed with the main obstetrical adverse outcomes: preeclampsia, UIGR, and preterm birth.
The level of glycosylated hemoglobin (HbA1c) was evaluated by Bio-Rad D-10. Blood pressure was measured manually according to the recommendations of ISSHP. Proteinuria level was detected using 24-h urine collections. Ultrasound investigations were performed by a standard procedure using Voluson E8 Expert (General Electric Medical Systems, USA).
Placental tissue was collected from the women undergoing spontaneous birth or caesarean section. Tissue samples from the central and marginal parts (1 cm3) were routinely fixed in 10% buffered formalin, included in paraffin and serially sectioned for the histological and the immunohistochemical analysis (IHC). Five-micron-thick sections were then cut and placed on charged poly-lysine coated slides (Thermo Fisher Scientific, USA). The sections were deparaffinized, rehydrated in graded alcohols, and further processed for staining. For histological analysis routine procedure, hematoxylin and eosin (H&E) stain was used. For immunostaining, the sections were first submitted to antigen retrieval by incubation in citrate buffer (pH 6.0) for 25 min at 96 ℃, the slides were subsequently incubated in a protein block for 30 min to block the nonspecific immunoreactivity. The primary monoclonal mouse antibody to kisspeptin (KISS1 ab55384, 1:150, Abcam, UK) and KISS1R (ab140839, 1:350, Abcam, UK) was layered over the section and incubated for 60 min at room temperature. The sections were then incubated with secondary antibodies Alexa Fluor 488 and 647 (1:1000, Abcam, UK) for 30 min in the dark. Nuclear counter-staining was realized with DAPI A4099 (AppliChem, USA). The slides were then enclosed in Fluorescent Mounting Medium (Dako, USA). A negative control without primary antibodies and positive control (first-trimester trophoblast) were done.
The morphometric investigation was carried out using a system of computer analysis of microscopic images, which includes a confocal microscope Fluo View 1000 (Olympus, Japan). Moreover, software FW10 was employed for the acquisition of images. Software Morphology 5.2 (Videotest, Russia) was used to measure the staining area, which was calculated as the ratio of the area of dyed tissue to the total tissue in the field of view (in %).
Statistical analyses
Statistical tests were performed using SPSS software (Statistical Packages for Social Sciences version 23, IBM). For the evaluation of sample distribution, the Kolmogorov–Smirnov test was performed. The mean value was calculated with 95% confidence interval (95% CI). The Kruskal–Wallis (one-way ANOVA) or Mann–Whitney U test was used for multiple group comparisons followed by the Dunn’s post hoc test to examine significant differences. Spearman’s rank correlation coefficient examined the relationships between the placental kisspeptin expression and clinical and obstetric outcomes. The correlation coefficient value (r) was either positive (direct correlation) or negative (inverse correlation), with values < 0.3 representing no correlation, 0.3 < 0.5 weak correlation, 0.5 < 0.7 moderately strong correlation, and > 0.7 strong correlation. Statistical significance was assumed at p values < 0.05.
Results
Characteristics of participants
The total of 65 pregnant women was recruited. There were no significant differences between the groups with respect to their age (Table 1). But the tendency to the higher age in the T2DM and GDM groups was observed. The maternal body mass index was higher in the T2DM + PE (33.4 kg/m2) and GDM + PE (28.5 kg/m2) patients, in comparison to those with PE (22.4 kg/m2), T1DM (23.1 kg/m2), and the control group (21.3 kg/m2). The mean gestational age of delivery was lower in pregnancies complicated by PE (36.3 ± 1.8 weeks) and T1DM (36 ± 0.8 weeks) compared to the healthy control group (39.5 ± 0.9 weeks) (p < 0.05). The rate of cesarean section was higher in PE and DM + PE groups compared to the other groups. All the groups showed no significant differences in the baby weight (p < 0.05). There were no differences between parity among the groups. The average level of HbA1c was higher in diabetic groups compared to the control and PE (Table 1). Cases of preterm birth were observed only in the T1DM and PE groups. Most of them were after 36 weeks of pregnancy (Table 1). One case of fetal IUGR was observed in the T2DM and GDM groups, and four (26.7%) in preeclampsia. All the demographic and clinical data are summarized in Table 1.
Localization and expression of KISS1 and KISS1R in preeclamptic and diabetic placentae
Immunohistochemical method was performed to examine the expression of the placental protein kisspeptin. KISS1/KISS1R immunolabelings were detected in all the placental samples. The expression of these markers was observed in the cell layers of the syncytiotrophoblast and villous cytotrophoblast (Fig. 1).
Placental expressions of KISS1 and KISS1R in diabetic pregnancy, preeclampsia and normal term pregnancy. Immunostaining predominantly present in syncytiotrophoblast and cytotrophoblast cells. Positively labeled cells appear green and red, magnification × 200. Red arrows indicate the syncytiotrophoblast; blue arrows indicate the cytotrophoblast
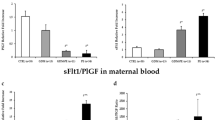
There were significant differences in the staining area of KISS1 among the study groups. The highest KISS1 immunostaining was observed in the syncytio and cytotrophoblast layers of preeclampsia and GDM groups in comparison to healthy pregnancies (Table 2, Fig. 2). The placental protein expression of KISS1 in groups with pre-gestational types of diabetes and preeclampsia was higher compared to the samples with DM no PE cohorts. In type-1 and -2 diabetes without PE, the placental staining of KISS1 was lower compared to the PE and GDM, but higher than in the control group (Table 2, Fig. 2). In the GDM cohorts, significant differences of KISS1 expression between the groups were not observed.
Placental KISS1R staining area was detected to be higher in case of pathology than in normal pregnancy (Table 2, Figs. 1, 2). KISS1R protein expression was higher in pre-gestational diabetes, gestational diabetes, and PE placental samples compared to standard term pregnancy (Table 2, Fig. 2). However, significant differences were detected only for T1DM and preeclampsia groups.
The next step of investigation was to explore the correlation links between the placental expression of KISS1/KISS1R and the main obstetric outcomes. We detected a strong direct link between the placental expressions of KISS1 (r = 0.81, p = 0.0001), KISS1R, and preeclampsia (r = 0.56, p = 0.001). Low correlation links were found between the placental expression of KISS1 and cases of IUGR (r = 0.29, p = 0.049) and preterm birth (r = 0.24, p = 0.046). The same trend was detected for the kisspeptin-1 receptor (Table 3). A strong inverse correlation link was observed between the KISS1 and preterm birth weight (r = − 0.73, p = 0.008). We did not find any significant correlations between the placental expressions of KISS/KISS1R and placental weight or HbA1c levels (Table 3).
Discussion
The normal trophoblast cell invasion and migration are crucial to the adequate development of the placenta. Alteration of these processes from the early stage leads to ischemia, reduction of uteroplacental flow, and impairs the structure and function of the placenta. Nowadays, the exploration of new biomarkers that can affect this mechanism attracts a lot of attention. The increased level of KISS1/KISS1R expression may play an influential role in the inhibition of trophoblast cell invasion [8, 16]. As a result, it leads to the inadequate transformation of the spiral arteries that are accepted as a cornerstone in the pathophysiology of PE.
The origin of PE is still unclear, but most of the investigations highlight the pathogenetic differences between the pre-existing and gestational co-morbidities and vascular diseases [17, 18]. It is known that the pre-gestational types of diabetes exist before the pregnancy and are associated with vascular and endothelial dysfunction. These changes can affect many biological processes in the developing placenta: impair angiogenesis, structure, function, and production of various placental biomarkers [12]. On the contrary, the patients with GDM are characterized by a mild form of hyperglycemia and associated with more placental dysfunction, but not structural pathology [19].
In our study, we demonstrated that the placental expression of KISS1 and KISS1R is higher in case of PE and diabetic pregnancy compared to the control group. Unexpectedly, we observed that in case of type-1 and -2 DM the expression of KISS1 was slightly lower compared to GDM and PE groups. It seems a little controversial taking into consideration that the vasculopathy, which is a character for pre-gestational types of DM, plays a significant role in the inhibition of trophoblast invasion. The possible explanation of these findings is the influence of another co-factor such as obesity, gestational weight gain, and the mode of delivery. These factors can affect the placental function and composition in diabetic pregnancy [19].
In patients with diabetes and PE, increased placental expression of KISS1/KISS1R was associated with adverse perinatal outcome. We established that the expression of KISS1/KISS1R correlates positively with signs and severity of PE. Additionally, we detected another positive and negative correlation links between the placental expression of KISS1/KISS1R and placental insufficiency and birth weight in case of preterm delivery. We did not establish any correlations between the kisspeptins and levels of glycosylated haemoglobin and placental weight, which is in concordance with other reports [20].
The present results agree with reports concerning the connection between the KISS1/KISS1R and development of PE and IUGR. Several studies have shown elevated KISS1 mRNA and protein expression in the placenta in case of PE. In the study of Vasquez-Alaniz et al. [21], a higher expression of KISS1 was shown in the placenta of women who had PE compared to the normal group. Meanwhile, this result was confirmed by Zhang et al. [22]. Matjila et al. (2016) reported high placental kisspeptin expression, but low circulating serum kisspeptin levels in pregnancies complicated by PE [23].
Regarding KISS1R (GPR-54), no difference was found between the healthy and preeclamptic pregnancies [23]. Interestingly, Qiao et al. found that the increased expression of KISS1 at both RNA and protein levels in the placenta was observed only in case of early PE. Additionally, they did not find any difference in placental KISS1R mRNA and protein expression between the normal and preeclamptic placentas [24].
Despite this, a few studies reported a decreased level of KISS1 expression in the placenta of pregnancies complicated by PE. Cartwright et al. (2012) assessed protein and mRNA levels of KISS1 and KISS1R in PE and normal term pregnancy groups. They found that in preeclamptic patients, the protein and mRNA placental expressions were reduced, but KISS1R was higher compared to the normal group [7]. The same results were observed in an earlier study by Qiao et al. (2005) [25].
There are only a few studies concerning placental kisspeptin expression in women with different types of diabetes during pregnancy. In an experimental study, Loegl et al. (2017) showed a significant increase in the gene expressions of KISS1 in trophoblast cells in the placenta from women with GDM [26]. Cetcovic et al. (2012) found that in pregnant women with T1DM and patients with gestational hypertension serum kisspeptin levels were significantly lower compared to the control group in all trimesters. In case of GDM and PE, the decrease of serum kisspeptin level was observed only in the second and third trimesters. They confirmed the suggestion that low levels of kisspeptins are associated with adverse perinatal outcome [20].
There are a few possible limitations of the present study. The total sample size in diabetic groups is relatively small. There are differences in time and mode of delivery between the normal pregnancy and the study groups. Our study only included patients with late-onset PE (> 34 weeks of gestation), which can affect the final results. The strong point of this study is the comparison of the placental expression of KISS1/KISS1R in pre-gestational DM, GDM, and PE groups.
The study shows altered expression of KISS1 and its receptor KISS1R in patients with DM and PE suggesting their role in the pathophysiological changes that occur in this disorder. KISS1 may be a perspective biomarker for the prediction of PE [27, 28]. Further studies are required to determine the exact role and amount of kisspeptins in the placenta. It provides additional information in the understanding of the pathophysiology of PE.
References
Hiden U, Bilban M, Knöfler M, Desoye G (2007) Kisspeptins and the placenta: regulation of trophoblast invasion. Rev Endocr Metab Disord 8:31–39. https://doi.org/10.1007/s11154-007-9030-8
Gottsch ML, Clifton DK, Steiner RA (2009) From KISS1 to kisspeptins: an historical perspective and suggested nomenclature. Peptides 30:4–9. https://doi.org/10.1016/j.peptides.2008.06.016
Janneau J-L, Maldonado-Estrada J, Tachdjian G et al (2002) Transcriptional expression of genes involved in cell invasion and migration by normal and tumoral trophoblast cells. J Clin Endocrinol Metab 87:5336–5339. https://doi.org/10.1210/jc.2002-021093
Mead EJ, Maguire JJ, Kuc RE, Davenport AP (2009) Kisspeptins: a multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. Br J Pharmacol 151:1143–1153. https://doi.org/10.1038/sj.bjp.0707295
Ohtaki T, Shintani Y, Honda S et al (2001) Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613–617. https://doi.org/10.1038/35079135
Yan C, Wang H, Boyd DD (2001) KiSS-1 Represses 92-kDa type IV collagenase expression by down-regulating NF-κB binding to the promoter as a consequence of IκBα-induced block of p65/p50 nuclear translocation. J Biol Chem 276:1164–1172. https://doi.org/10.1074/jbc.M008681200
Cartwright JE, Williams PJ (2012) Altered placental expression of kisspeptin and its receptor in pre-eclampsia. J Endocrinol 214:79–85. https://doi.org/10.1530/JOE-12-0091
Bilban M, Ghaffari-Tabrizi N, Hintermann E et al (2004) Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci 117:1319–1328. https://doi.org/10.1242/jcs.00971
Horikoshi Y, Matsumoto H, Takatsu Y et al (2003) Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab 88:914–919. https://doi.org/10.1210/jc.2002-021235
Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R (2010) Pre-eclampsia. Lancet 376:631–644. https://doi.org/10.1016/S0140-6736(10)60279-6
Kawamura T, Kakogawa J, Takeuchi Y et al (2007) Measurement of placental oxygenation by transabdominal near-infrared spectroscopy. Am J Perinatol 24:161–166. https://doi.org/10.1055/s-2006-958155
Nelson SM, Coan PM, Burton GJ, Lindsay RS (2009) Placental structure in type 1 diabetes: relation to fetal insulin, leptin, and IGF-I. Diabetes 58:2634–2641. https://doi.org/10.2337/db09-0739
Desoye G, Hauguel-de Mouzon S (2007) The Human placenta in gestational diabetes mellitus: the insulin and cytokine network. Diabetes Care 30:S120–S126. https://doi.org/10.2337/dc07-s203
Metzger BE (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33:676–682. https://doi.org/10.2337/dc09-1848
Brown MA, Magee LA, Kenny LC et al (2018) The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 13:291–310. https://doi.org/10.1016/j.preghy.2018.05.004
Roseweir AK, Katz AA, Millar RP (2012) Kisspeptin-10 inhibits cell migration in vitro via a receptor-GSK3 beta-FAK feedback loop in HTR8SVneo cells. Placenta 33:408–415. https://doi.org/10.1016/j.placenta.2012.02.001
Huppertz B (2008) Placental origins of preeclampsia. Hypertension 51:970–975. https://doi.org/10.1161/HYPERTENSIONAHA.107.107607
Raymond D, Peterson E (2011) A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv 66:497–506. https://doi.org/10.1097/OGX.0b013e3182331028
Colomiere M, Permezel M, Riley C et al (2009) Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur J Endocrinol 160:567–578. https://doi.org/10.1530/EJE-09-0031
Ćetković A, Miljic D, Ljubić A et al (2012) Plasma kisspeptin levels in pregnancies with diabetes and hypertensive disease as a potential marker of placental dysfunction and adverse perinatal outcome. Endocr Res 37:78–88. https://doi.org/10.3109/07435800.2011.639319
Vazquez-Alaniz F, Galaviz-Hernandez C, Marchat LA et al (2011) Comparative expression profiles for KiSS-1 and REN genes in preeclamptic and healthy placental tissues. Eur J Obstet Gynecol Reprod Biol 159:67–71. https://doi.org/10.1016/j.ejogrb.2011.07.019
Zhang H, Long Q, Ling L et al (2011) Elevated expression of KiSS-1 in placenta of preeclampsia and its effect on trophoblast. Reprod Biol 11:99–115. https://doi.org/10.1016/S1642-431X(12)60048-5
Matjila M, Millar R, van der Spuy Z, Katz A (2016) Elevated placental expression at the maternal–fetal interface but diminished maternal circulatory kisspeptin in preeclamptic pregnancies. Pregnancy Hypertens An Int J Women’s Cardiovasc Heal 6:79–87. https://doi.org/10.1016/j.preghy.2015.11.001
Qiao C, Wang C, Zhao J et al (2012) Elevated expression of KiSS-1 in placenta of chinese women with early-onset preeclampsia. PLoS ONE 7:e48937. https://doi.org/10.1371/journal.pone.0048937
Qiao C, Cheng D-L, Zhang S-L et al (2005) The role of KiSS-1 and matrix metalloproteinase-9 in regulation of invasion of trophoblasts. Zhonghua Yi Xue Za Zhi 85:839–842
Loegl J, Nussbaumer E, Cvitic S et al (2017) GDM alters paracrine regulation of feto-placental angiogenesis via the trophoblast. Lab Investig 97:409–418. https://doi.org/10.1038/labinvest.2016.149
Armstrong RA, Reynolds RM, Leask R et al (2009) Decreased serum levels of kisspeptin in early pregnancy are associated with intra-uterine growth restriction and pre-eclampsia. Prenat Diagn 29:982–985. https://doi.org/10.1002/pd.2328
Smets EML, Deurloo KL, Go ATJI et al (2008) Decreased plasma levels of metastin in early pregnancy are associated with small for gestational age neonates. Prenat Diagn 28:299–303. https://doi.org/10.1002/pd.1969
Acknowledgements
We thank all the patients who participated in this study and staff who assisted with sample collection.
Author information
Authors and Affiliations
Contributions
KRV: Project development, Manuscript writing, Data analysis. DAO: Project development, Data Collection. AEN: Data analysis, Data collection. OAR: Data collection. AON: Manuscript editing. PVO: Manuscript editing. KIM: Manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The local ethics committee approved the presented study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kapustin, R.V., Drobintseva, A.O., Alekseenkova, E.N. et al. Placental protein expression of kisspeptin-1 (KISS1) and the kisspeptin-1 receptor (KISS1R) in pregnancy complicated by diabetes mellitus or preeclampsia. Arch Gynecol Obstet 301, 437–445 (2020). https://doi.org/10.1007/s00404-019-05408-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05408-1