Abstract
Purpose
To compare the clinical outcome of fresh embryo transfer with frozen-thawed embryo transfer in subsequent cycle of GnRH antagonist protocol.
Methods
Totally, 1430 women were enrolled from the cases of our Assisted Reproduction Center from January 2015 to January 2019 for this retrospective cohort study. The inclusion criteria of the subjects included women with ages under 40 years, 3–10 oocytes retrieved, good embryo quality according to gardener score, GnRH antagonist protocol, underwent first cycle of fresh embryo transfer or freeze-all strategy and transferred in subsequent cycle. However, the patients with endometriosis, PGD/PGS cycles, history of recurrent pregnancy loss and uterine pathology were excluded. 495 women of group I underwent fresh embryo transfer in first cycle and 935 patients of group II received frozen-thawed transfer in subsequent cycle. The primary outcome was clinical pregnancy rate. A logistic regression analysis was performed to determine the variables that could be independently associated with clinical pregnancy rate. Models were adjusted for covariates including patients’ age, fertilization type, infertility type, infertility duration, the number of oocytes retrieved, the number of embryos transferred and type of embryo transferred.
Results
Clinical pregnancy rate was significantly higher in frozen-thawed embryo transfer than in fresh embryo transfer (63.70% vs. 54.50%, p < 0.001). Miscarriage rate and ectopic pregnancy rate were comparable between two groups. Variables independently associated with clinical pregnancy rate were fresh/frozen embryo transfer, patients’ age and the number of embryos transferred. After adjusting for variables, the frozen embryo transfer [adjusted odds ratio (aOR) 0.75; 95% CI, 0.59–0.95, p = 0.016] was a predictive factor of clinical pregnancy rate.
Conclusion
Frozen embryo transfer is better than fresh embryo transfer in GnRH antagonist cycle in women with 3–10 oocytes retrieved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gonadotropin-releasing hormone (GnRH) antagonist protocols have been the mainstay in in vitro fertilization (IVF) treatment, minimizing the risk of ovarian hyperstimulation syndrome (OHSS) with the use of GnRH agonist (GnRHa) for oocyte maturation. However, the luteolysis following GnRHa trigger is associated with luteal phase defect which could in turn decrease clinical pregnancy rate. The potential adverse effects of GnRH antagonists on endometrial quality may be detrimental to the pregnancy outcome. Controlled ovarian hyperstimulation (COH) may also impair endometrial receptivity in IVF cycles [1, 2]. Therefore, embryo cryopreservation has become a routine procedure in some IVF centers, the “freeze-all” policy has been proven as a safe and advantageous alternative for OHSS prevention and restoring endometrial receptivity.
In frozen-thawed embryo transfer (FET) cycles, embryos are transferred into a more physiological environment, resulting in better pregnancy outcome and lower maternal and perinatal morbidity as compared to fresh embryo transfer [3]. The results can probably be explained by the improvement of embryo-endometrium synchrony. Another possible mechanism is the up-regulated progesterone receptor expression associated with COH in fresh cycles.
However, there are controversy regarding the risks and benefits and patient selection of the freeze-all policy. To our knowledge, no noninvasive methods are effective to evaluate endometrium receptivity. First, there were no sufficiently convincing evidences on the cost-effectiveness of FET. Additionally, other arguments should be taken into account, namely extra cost and time, longer time to live birth and stricter cryopreservation techniques. Second, although obstetrical and perinatal outcomes seemed to be better following FET, it is also related to higher incidence of large for gestational age (LGA) [4]. Lastly, although cryopreservation techniques have been effective after improvement, embryo cryo-damage is not evitable.
The aim of the present study was to compare clinical pregnancy rate between fresh embryo transfer and freeze-all strategy plus transfer in subsequent cycle in GnRH antagonist protocol.
Materials and methods
Study design and patients
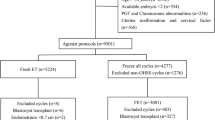
This was a retrospective cohort study in which the IVF is practiced from January 2015 to January 2019 in the Center for Assisted Reproductive Technology of Northwest Women’s and Children’s Hospital, China. Data were extracted from clinical records. Women patients were eligible if they met the following criteria: (1) ages under 40 years (2) 3 and 10 oocytes retrieved (3) good embryo quality according to gardener score (4) GnRH antagonist protocol (5) underwent first cycle of fresh embryo transfer or freeze-all strategy and transfer in subsequent cycle. The exclusion criteria were: (1) endometriosis, (2) PGD/PGS cycles, (3) history of recurrent pregnancy loss, and (4) uterine pathology. Totally 1430 women patients were included in this study, in which 495 and 935 subjects received fresh embryo transfer in group I and frozen embryo transfer in group II, respectively.
IVF and fresh embryo transfer
In our Assisted Reproduction Center, the COH with gonadotropin started from day 2 of menses using recombinant follicle-stimulating hormone (r-FSH, Gonal F, Merck Serono, Italy) at an initial dose of 150–300 IU per day. GnRH antagonist was added when follicle diameter reached 14 mm. The r-hCG trigger was administrated when at least two leading follicles reached a diameter of 18 mm. Oocytes were retrieved 36 h later. Conventional IVF or intracytoplasmic sperm injection (ICSI) was performed after oocyte retrieval. All patients were assigned into two groups (as shown in Tables 1 and 2) by doctors’ preference. In group I, one to two high-quality fresh embryos were transferred into the uterus of women free of OHSS, high progesterone level (> 1.5 ng/ml) on human chorionic gonadotropin (hCG) trigger day, then the spare embryos were cryopreserved for FET later on. In group II, all the embryos were frozen and patients underwent FET in the subsequent cycle.
Endometrial preparation for FET
Before FET in group II, the endometrial priming started on the fifth day of menstrual cycle and estradiol valerate was orally administered at a dose of 6 mg daily. After 10–12 days of the endometrial preparation, the transvaginal ultrasonography and progesterone level test were performed. If the endometrial thickness was over 8 mm and progesterone level under 1.5 ng/ml, progesterone was administrated.
Luteal phase support and FET
In group I, the luteal support was administered using vaginal progesterone (90 mg q.d.; Crinone, Serono, Hertfordshire, UK), or vaginal progesterone soft capsules (0.2 g t.i.d; Utrogestan, Besins, France), or intramuscular progesterone (60 mg q.d.; Zhejiang, Xianju, China), oral progesterone (10 mg t.i.d.; Dydrogesterone, Abbott Biologicals B.V., Netherlands), and by parallel administration of estradiol valerate (2 mg b.i.d.; Progynova, Bayer, France) until the confirmation of biochemical pregnancy, and the luteal support was maintained to week 10 of gestation.
In group II the participants received estradiol valerate orally at 4–6 mg/day from day 3–5 of subsequent artificial menstrual cycle. Vaginal progesterone (90 mg q.d.; Crinone, Serono, Hertfordshire, UK), or vaginal progesterone soft capsules (0.2 g t.i.d; Utrogestan, Besins, France) or intramuscular progesterone (60 mg q.d.; Zhejiang, Xianju, China), and oral progesterone (10 mg t.i.d.; Dydrogesterone, Abbott Biologicals B.V., Netherlands) were added if the endometrial thickness is ≥ 8 mm. Frozen embryos were thawed and one or two high-quality embryos were transferred into the uterus 4 days (D3 embryos) or 6 days (D5 embryos) in the subsequent cycle after the progesterone initiation.
All patients were followed up and pregnancy confirmed with biochemical hCG test 14 days after embryo transfer. If serum hCG was above 50 IU/l, luteal support maintained to 10 weeks of gestation. Transvaginal ultrasonography was performed 5 weeks after embryo transfer.
Definition of clinical outcomes
Clinical pregnancy was defined as a pregnancy diagnosed by ultrasonographic visualization of one or more gestational sacs or definitive clinical signs of pregnancy. Ectopic pregnancy was defined as gestational sac observed by ultrasound outside the uterine cavity. Miscarriage was defined as spontaneous loss of a clinical pregnancy before 22 completed weeks of gestational age.
Statistical analysis and different models of adjustment
The descriptive data on participant characteristics were summarized using the mean and standard deviation for continuous variables. Counts and proportions were used for the categorical variables. Chi-square tests were performed to compare the categorical variables. The analysis of covariance (normally distributed variables) or a Kruskal–Wallis test (abnormally distributed variables) was used to compare the continuous variables of different groups. Univariate and multivariable conditional logistic regression were used to analyze the relationship between timing of transfer in different models. In crude model we did not adjust any covariates. In model I we only adjusted the patients’ age. In model II we adjusted female patients’ age, fertilization type, infertility type, infertility duration, the number of oocytes retrieved, the number of embryos transferred and D3/D5 embryo transfer. All statistical analyses were performed with SPSS Version 13.0 and the level of significance was set at p < 0.05.
Results
The baseline characteristics of groups I and II are presented in Table 1. Patients’ age, infertility duration, body mass index (BMI), and antral follicle count (AFC) were all comparable between two groups (each at p > 0.05). There were no differences in percentages of different fertilization types and infertility types, days (D3/D5) of embryo transfer between two groups (each at p > 0.05). Notably, the number of oocytes retrieved in fresh embryo transfer was higher than that in FET (p < 0.05). The percentages of the number of embryo transferred were higher in fresh embryo transfer than those in FET (p < 0.001). However, the clinical pregnancy rate in fresh embryo transfer was significantly lower than that in FET (54.50% vs. 63.70%, p < 0.001). Miscarriage rate and ectopic pregnancy rate were all comparable between two groups (each at p > 0.05).
Univariate analysis for clinical pregnancy rate is shown in Table 2. FET (OR 0.68; 95% CI 0.55–0.85) was a predictive factor of clinical pregnancy rate compared to fresh embryo transfer (p < 0.001). Double embryo transfer was a predictive factor of clinical pregnancy rate compared to that with single embryo (p < 0.001). Patients’ age (OR 1.06; 95% CI 1.03–1.09) was a risk factor of clinical pregnancy rate compared to fresh embryo transfer (p < 0.001). Fertilization type, infertility type, the number of oocytes retrieved, ages of embryos for transfer had no association with clinical pregnancy rate.
The relationship between timing of transfer in different models is shown in Table 3. After adjusting for patients’ age, fertilization type, infertility type, infertility duration, the number of oocytes retrieved, the number of embryos transferred and type of embryo transferred, we found FET was a predictive factor (OR 0.75; 95% CI 0.59–0.95) compared to fresh embryo transfer (p = 0.016).
Discussion
The main finding of this large, retrospective, cohort study was that freeze-all policy was advantageous in IVF outcomes, compared with fresh embryo transfer. GnRH antagonist protocol is effective in preventing premature LH surge and, therefore, can result in a more cost-effective ovarian stimulation compared to GnRHa protocol [5]. GnRH antagonist protocol requires shorter duration of ovulation induction and less gonadotropins used. In recent years, there was a shift toward increased use of FET, coincident with faster increase of live birth rate in FET than in fresh embryo transfer [2]. Some studies have shown implantation rates are reduced in GnRH antagonist-stimulated cycles, which is in accordance with our study [1, 6]. However, some studies indicate similar outcome between fresh embryo transfer and FET [7].
By evaluating outcome in GnRH antagonist protocol generated via both fresh embryo transfer and FET, the effect of GnRH antagonist on the endometrium of women has been indirectly evaluated. It leads to suggestion that a potentially deleterious effect of GnRH antagonist on the endometrium may contribute to the lower pregnancy rate in fresh embryo transfer cycles. At the time of transfer of thawed embryos, the influence of GnRH antagonist no longer exist, despite the dose and duration of GnRH antagonist used [8]. However, some studies suggested that GnRH antagonists do not negatively affect oocyte and embryo quality [9].
Controlled ovarian stimulation is routinely used to promote multi-follicle growth in IVF cycle. The developing follicles produce supraphysiologic levels of estradiol and progesterone level. Estradiol and progesterone are closely linked to endometrial development and receptivity. Studies have shown a structural histological advancement of the endometrium and an absence of endometrial pinopods at the time of embryo implantation in gonadotropin-stimulated cycles [10,11,12]. The histologic advancement of endometrium correlates with premature progesterone rise and eventually leads to implantation failure due to embryo-endometrium asynchrony [13,14,15].
Some studies including two meta-analysis indicate that FET is associated with significantly reduced risks of preterm birth, small gestational age (SGA), low birth weight (LBW), perinatal mortality, placental abruption and placenta previa [16,17,18,19,20,21].
The limitation of the present study was a retrospective cohort study from a single center. Additionally, although we used multivariable logistic regression to control confounders between the different groups, the findings of our study might be potentially confounded by unmeasured or unidentified covariates.
Although our study was retrospective, our results show that there were no significant differences between fresh embryo transfer and frozen embryo transfer in baseline characteristics except for number of oocyte retrieved and number of embryo transferred, indicating the homogeneity and good comparability of the two groups. In addition, only patients who did not transfer in the fresh cycle due to GnRH antagonist protocol were included in the group of FET. In this way, potential bias because of other reason of cancelling fresh embryo transfer was avoided. Furthermore, we excluded women who had embryo transfer in fresh cycle in group II, since women who failed in the fresh embryo transfer may have worse clinical outcome. This can avoid selecting bias.
In conclusion, the outcome of GnRH antagonist protocol can be improved using freeze-all policy in women with 3–10 oocytes retrieved. Further multi-center prospective randomized clinical trials are needed to confirm the advantage of freeze-all policy.
References
Shapiro BS, Daneshmand ST, Garner FC (2011) Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril 96(2):344–348
Shapiro BS, Daneshmand ST, Garner FC (2014) Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril 102(1):3–9
Evans J, Hannan NJ, Edgell TA (2014) Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update 20(6):808–821
Pelkonen S, Koivunen R, Gissler M (2010) Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995–2006. Hum Reprod 25(4):914–923
Depalo R, Jayakrishan K, Garruti G (2012) GnRH agonist versus GnRH antagonist in in vitro fertilization and embryo transfer (IVF/ET). Reprod Biol Endocrinol 10(4):26
Roque M, Valle M, Guimaraes F (2015) Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril 103(5):1190–1193
Aflatoonian AM-TM, Mojtahedi MF (2018) Fresh versus frozen embryo transfer after gonadotropinreleasing hormone agonist trigger in gonadotropinreleasing hormone antagonist cycles among high responder women: a randomized, multi-center study. Int J Reprod Biomed (Yazd) 16(1):9–18
Kol S, Lightman A, Hillensjo T (1999) High doses of gonadotrophin-releasing hormone antagonist in in-vitro fertilization cycles do not adversely affect the outcome of subsequent freeze-thaw cycles. Hum Reprod 14(9):2242–2244
Zikopoulos K, Kolibianakis EM, Camus M (2004) Duration of gonadotropin-releasing hormone antagonist administration does not affect the outcome of subsequent frozen-thawed cycles. Fertil Steril 81(2):473–475
Garcia JE, Acosta AA, Hsiu JG (1984) Advanced endometrial maturation after ovulation induction with human menopausal gonadotropin/human chorionic gonadotropin for in vitro fertilization. Fertil Steril 41(1):31–35
Kolb BA, Paulson RJ (1997) The luteal phase of cycles utilizing controlled ovarian hyperstimulation and the possible impact of this hyperstimulation on embryo implantation. Am J Obstet Gynecol 176(6):1262–1267
Mirkin S, Nikas G, Hsiu JG (2004) Gene expression profiles and structural/functional features of the peri-implantation endometrium in natural and gonadotropin-stimulated cycles. J Clin Endocrinol Metab 89(11):5742–5752
Ubaldi F, Bourgain C, Tournaye H (1997) Endometrial evaluation by aspiration biopsy on the day of oocyte retrieval in the embryo transfer cycles in patients with serum progesterone rise during the follicular phase. Fertil Steril 67(3):521–526
Nikas G, Develioglu OH, Toner JP (1999) Endometrial pinopodes indicate a shift in the window of receptivity in IVF cycles. Hum Reprod 14(3):787–792
Kolibianakis E, Bourgain C, Albano C (2002) Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril 78(5):1025–1029
Maheshwari A, Pandey S, Shetty A (2012) Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril 98(2):368–377
Ishihara O, Araki R, Kuwahara A (2014) Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril 101(1):128–133
Wennerholm UB, Henningsen AK, Romundstad LB (2013) Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod 28(9):2545–2553
Wennerholm UB, Soderstrom-Anttila V, Bergh C (2009) Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod 24(9):2158–2172
Pinborg A, Wennerholm UB, Romundstad LB (2013) Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update 19(2):87–104
Johnson KM, Hacker MR, Resetkova N (2019) Risk of ischemic placental disease in fresh and frozen embryo transfer cycles. Fertil Steril 111(4):714–721
Acknowledgements
We thank the staff from Northwest Women’s and Children’s Hospital for their assistance with the data collection. We thank all participants in this study.
Funding
The study was funded by National Natural Science Foundation of China (No. 81771657 https://www.nsfc.gov.cn/) and General Projects of Social Development in Shaanxi Province (No. 2018SF-260).
Author information
Authors and Affiliations
Contributions
XTL: manuscript writing, HYB: data analysis, WHS: manuscript writing, JZS: protocol development.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
The approval of the Institutional Review Board of Northwest Women’s and Children’s Hospital was obtained for this retrospective cohort study (Number 2018002). All research was performed in accordance with relevant guidelines and regulations.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Bai, H., Shi, W. et al. Frozen-thawed embryo transfer is better than fresh embryo transfer in GnRH antagonist cycle in women with 3–10 oocytes retrieved: a retrospective cohort study. Arch Gynecol Obstet 300, 1791–1796 (2019). https://doi.org/10.1007/s00404-019-05373-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05373-9