Abstract
Purpose
To compare the clinical and ongoing pregnancy rates between a protocol using oral dydrogesterone with human menopausal gonadotropin (HMG) for progestin-primed ovarian stimulation (PPOS) and the typical gonadotropin-releasing hormone (GnRH) antagonist regimen in women undergoing controlled ovarian hyperstimulation (COH).
Methods
This was a prospective, controlled study of 251 women who underwent COH for in vitro fertilization between October 2016 and July 2017. The patients were allocated alternately into two groups: a dydrogesterone protocol (study group) and a GnRH antagonist protocol (control group). In study group, dydrogesterone (20 mg/day) plus HMG (150 or 225 IU) were administered simultaneously beginning on days 2 or 3 of the menstrual cycle. In both groups, all high-quality embryos were cryopreserved for later transfer. The primary outcome was the ongoing pregnancy rate at 12 weeks per frozen–thawed embryo transfer (FET) and the secondary outcome was the clinical pregnancy rate.
Results
None of the patients experienced a premature luteinizing hormone surge. During the follow-up period, 397 FET cycles were completed. The ongoing pregnancy rates at 12 weeks were 40.0% in study group versus 38.1% in control group (absolute difference 1.9%; 95% CI − 6.83 to 17.2%). The clinical pregnancy rate in study group (52.8%) was also not inferior to that in control group (49.5%; absolute difference 3.3%; 95% CI − 4.02 to 20.2%).
Conclusions
The clinical and ongoing pregnancy rates in study group were comparable to those in control group. Therefore, PPOS with dydrogesterone is a reasonable option to provide COH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During controlled ovarian hyperstimulation (COH), suppressing the premature luteinizing hormone (LH) surge is important for good outcomes in assisted reproductive technology (ART). The protocols for COH generally use gonadotropin-releasing hormone (GnRH) agonists and antagonists to prevent an endogenous LH surge from occurring before follicular maturation [1]. The most recommended protocols for COH are the use of a GnRH antagonist protocol, a GnRH agonist trigger, and freezing all embryos with later transfer to prevent ovarian hyperstimulation syndrome (OHSS) [2]. GnRH antagonists are effective for rapid and reversible suppression of LH release. Therefore, using a GnRH agonist trigger for the final oocyte maturation can prevent severe OHSS [3]. However, the conventional GnRH antagonist protocol is expensive and some patients experience a premature LH surge, resulting in undesired ovulation [4]. If an LH surge occurs and some follicles ovulate before oocyte retrieval, the cycle cancellation rate rises. Therefore, new protocols need to be established with improved efficacy and for the patients’ convenience to effectively prevent a premature LH surge.
The previous studies have demonstrated that the administration of several progestins during COH can effectively suppress a premature LH surge [5,6,7,8]. Progesterone (P4), which is secreted by the corpus luteum, strongly inhibits pulsatile GnRH and LH secretion, and prevents estradiol (E2)-induced positive feedback effects during the luteal phase. High baseline P4 levels in COH have no negative effect on oocyte/embryo quality [9, 10]. In the last decades, when IVF relied on fresh embryo transfer, high P4 levels on the day of trigger had a negative effect on endometrial receptivity. Currently, superior quality of cryopreserved embryos and precise thawing are possible by advanced vitrification techniques, and in vitro fertilization (IVF) no longer requires the transfer of fresh embryos. The “freeze-all” strategy can increase cumulative pregnancy rates, and decrease multiple pregnancy rates, ectopic pregnancy rates, and the risk of OHSS [11, 12].
Only a few studies have reported the efficacy of a combination of dydrogesterone and human menopausal gonadotropin (HMG) for progestin-primed ovarian stimulation (PPOS) in IVF cycles [8, 13]. Dydrogesterone is a synthetic progestin that is closely related to endogenous P4 in its molecular structure and its pharmacological effects. Moreover, dydrogesterone does not interfere with the measurement of endogenous P4 production. Dydrogesterone has less androgenic activity and enhanced oral bioavailability (28%) compared with other progestins, with a half-life of 8 h, and it is highly selective for the P4 receptor and has fewer adverse effects than other progestins [14,15,16]. The oral route of administration of dydrogesterone is thought to be a more patient-friendly regimen that might improve compliance with treatment. Therefore, here we compared the clinical and ongoing pregnancy rates for a COH protocol using oral dydrogesterone plus HMG (study group) with a standard GnRH antagonist protocol (control group).
Materials and methods
Study setting and allocation of patients
This prospective, controlled study was conducted at a private infertility clinic between October 2016 and July 2017, and was performed in accordance with the Declaration of Helsinki for medical research and Good Clinical Practice guidelines. The study protocol was also approved by the Ethics Committee of the Institutional Review Board of the clinic. Written informed consent was obtained from all of the enrolled patients. This trial has been registered with the UMIN Clinical Trial Registry in Japan (R000033778UMIN000029564).
Inclusion criteria were as follows: age younger than 41 years, anti-Müllerian hormone (AMH) levels greater than 1.00 ng/ml, and first or second cycle of IVF or intra-cytoplasmic sperm injection (ICSI) at the Kamiya Ladies Clinic. Patients who had endometriosis grade 3 or higher, documented cycles with no oocyte retrieved, and any contraindications for COH were excluded.
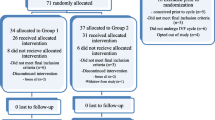
Patients were allocated consecutively to one of two COH groups in an alternating fashion. Odd-numbered patients were allocated to study group and even-numbered patients were assigned to control group in a non-blinded fashion. A total of 251 women were enrolled in the study, comprising 125 in study group and 126 in control group.
Study protocol
Controlled ovarian hyperstimulation
In study group, patients were administered HMG (Teizo®, 150 or 225 IU; ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) and dydrogesterone (Duphaston®, 20 mg; Abbott Healthcare, Tokyo, Japan) daily from days 2 or 3 of the menstrual cycle onward, following ultrasound confirmation of the absence of follicles larger than 10 mm. The initiating dose of 150 IU/day HMG was used for patients with an AMH level greater than 3.00 ng/ml or an antral follicle count > 15. A dose of 225 IU/day HMG was used for other patients.
In control group, patients were administered HMG (150 or 225 IU per day) on days 2 or 3 of the menstrual cycle. The choice of the initial HMG dose was decided in the same manner as for study group. When either the leading follicle reached 14 mm or serum E2 levels exceeded 1000 pg/ml, a GnRH antagonist (Ganirelix® 0.25 mg; MSD, Tokyo, Japan or Cetrotide® 0.25 mg; EMD-Serono, Tokyo, Japan) was administered by subcutaneous injection every 24 h to suppress premature LH surges following a flexible protocol.
In both groups, follicular monitoring using ultrasonography started on day 8 of the menstrual cycle. This monitoring was performed every 2 or 3 days, and a blood sample was taken at every visit to check serum follicle-stimulating hormone, LH, E2, and P4 concentrations. The HMG dose was increased by 75 IU when the speed of follicle growth was assessed as slow. When the main dominant follicle size was close to 20 mm in diameter, the final stage of oocyte maturation was triggered using a nasal spray of GnRH agonist (Buserecur®; Fuji Pharma, Tokyo, Japan). Patients were given a low dose of human chorionic gonadotropin (hCG; Gonatropin® 1000 IU; ASKA Pharmaceutical Co., Ltd. or hCG F® 2500 IU; Fuji Pharma) added as a co-trigger for ovulation only for those with hypothalamic–pituitary insufficiency or those with poor COH responses in the previous ART cycles. Patients who received the final trigger underwent transvaginal ultrasound-guided oocyte retrieval 35–37 h later. All follicles with diameters larger than 10 mm were aspirated.
Insemination of the aspirated oocytes was carried out in vitro, by either conventional insemination or ICSI, depending on the partner’s semen parameters. Embryos were examined for the number and regularity of blastomeres and the degree of embryonic fragmentation to assess quality on the 2nd or 3rd day of culture, according to published criteria [17]. One or two good quality embryos were frozen by vitrification on the 2nd or 3rd day after oocyte retrieval. The other embryos were placed in extended culture until they reached the blastocyst stage. At this point, only blastocysts with good morphology were frozen on days 5–7. Any instance of OHSS was defined according to a published classification system [18].
Endometrial preparation and frozen–thawed embryo transfer (FET)
The method of endometrial preparation was similar in both groups. A hormone replacement therapy (HRT) cycle was performed for all of the patients. They received dermal patches of 1.44 mg estradiol every other day (Estrana®; Hisamitsu Pharmaceutical Co., Ltd., Tokyo, Japan) and oral estradiol valerate 2.00 mg × 2 (Progynova®; Bayer, NSW, Australia) from days 2 or 3 of the menstrual cycle. The endometrial thickness was checked on cycle days 12–14 in patients undergoing a HRT cycle, and progestin capsules (500 mg P4 vaginal suppositories) and oral progestin (Duphaston®; 30 mg dydrogesterone) were administered daily in patients with an endometrial thickness > 7 mm. The transfer of day 3 embryos or blastocyst was scheduled based on embryo and endometrium synchronization. Once pregnancy was achieved (see below), the exogenous estrogen and progestin supplementation were continued until 10 weeks of gestation.
The primary outcome was defined as the ongoing pregnancy rate per FET cycle. The secondary outcomes were the numbers of cumulus–oocyte complexes (COCs) retrieved per COH cycle, the fertilization rate, the numbers of viable embryos, the clinical pregnancy rate, and the early miscarriage rate. Clinical pregnancy was defined as the presence of gestational sacs during an ultrasound examination up to 7 weeks of gestation. Ongoing pregnancy was defined as continuous fetal heartbeats as assessed by ultrasound at 12 weeks. The early miscarriage rate was defined as the proportion of pregnancies arresting before 12 weeks of gestation. Cycle cancellation refers to patients who completed oocyte retrieval without producing viable embryos.
Statistical analysis
According to the previous reports, the ongoing pregnancy rate of GnRH antagonist protocol was assumed to be 27–30% [3]. Therefore, control group was assumed to have a pregnancy rate of 30%. Study group was expected to have a pregnancy rate of ~ 40%. One hundred two patients per group were required to achieve at least 85% probability that the lower limit of the Wald 95% confidence interval (CI) for the difference of pregnancy rates exceeded − 10% (a non-inferiority margin of 10%). Given the possibility of a 10% dropout rate, we designed the study to include a total of 110 women in each group.
Data are shown as the mean ± standard deviation (SD). Statistical analysis was performed using StatFlex version 6.0 (Artech Co., Ltd., Osaka, Japan). The results were compared between the two groups using the Chi squared test, unpaired Student’s t test, or the Mann–Whitney nonparametric U test. P < 0.05 was considered statistically significant. The primary outcome analysis used a one-sided 95% CI with a non-inferiority margin of 10% for the difference in pregnancy rates between the two groups. The dydrogesterone protocol was declared non-inferior if the lower boundary of the 95% CI was less than 10% below that of the control. This margin was defined based on historical evidence of the active comparator (a well-established standard treatment). In clinical trials, given patient and treatment variability, a new treatment that performs within 10–20% of an old treatment is often the margin that used to be called non-inferior [19,20,21].
Results
Patients’ characteristics
Table 1 shows the patients’ characteristics, including age, body mass index, basal hormone profile, duration of infertility, and the indications for IVF/ICSI treatment. We treated couples with tubal infertility, endometrial infertility, male infertility, and idiopathic infertility. There were no significant differences in the incidences of these variables between the two groups. A total of 251 women completed oocyte retrieval and 397 completed FET cycles. Figure 1 shows an outline of this study.
Ovarian stimulation, follicular development, oocyte performance, and hormonal profile
The mean numbers of retrieved COCs (10.71 ± 6.56) and meiosis stage II (MII) oocytes (8.53 ± 5.39) in study group were similar to those of control group (8.71 ± 4.27 and 11.10 ± 5.19, respectively). Six patients in study group and five in control group had either unfertilized oocytes or poor-quality embryos. Table 2 shows the clinical and cycle outcomes of COH treatment in both groups. The duration of HMG administration and HMG doses in study group were significantly greater than those in control group (both P < 0.001). No significant difference were found in the numbers of retrieved COCs, the oocyte maturation rates, the fertilization rates, the cleavage rates, the viable embryo production rates, or the cycle cancellation rates between the two groups. The duration of restarting menstruation after oocyte retrieval was significantly shorter in study group than in control group (9.5 ± 2.2 days versus 12.2 ± 2.7 days, respectively; P < 0.001; Fig. 2). Restarting menstruation after oocyte retrieval early is favorable for preventing an early onset of OHSS. Although one patient in each group experienced moderate OHSS during the study period, both of them recovered within 7 days after oocyte retrieval without any treatment. The rate of moderate OHSS was similar between the groups. Thus, we could control the incidence of OHSS using these COH protocols.
The mean LH value on the trigger day was significantly lower in study group than in control group (P < 0.001; Table 2). No incidence of an LH surge was found in either group. Although two patients in each group experienced premature partial ovulation, oocyte retrieval was possible for the remaining follicles.
Pregnancy outcomes in FET cycles
During the 10 months of observation, 240 women in both groups completed a total of 397 FET cycles and 437 embryos were thawed. Among these FET cycles, single-embryo transfer was performed in 90.8% patients in both groups. The mean number of embryos per FET cycle was similar. The clinical pregnancy rate per transfer in study group was slightly higher than in control group, but this did not reach significance. These results indicated that embryos in study group shared better development potential than did those in control group (Table 3).
The primary outcome of this study, the ongoing pregnancy rate at 12 weeks of gestation was met, with the dydrogesterone protocol demonstrating non-inferiority to the GnRH antagonist protocol. The ongoing pregnancy rates at 12 weeks were 40.0% in study group versus 38.1% in control group (absolute difference 1.9%; 95% CI − 6.83 to 17.2%). The clinical pregnancy rate in study group (52.8%) was also not inferior to that in control group (49.5%; absolute difference 3.3%; 95% CI − 4.02 to 20.2%). Therefore, non-inferiority of the dydrogesterone protocol versus the GnRH antagonist protocol was demonstrated, because the lower boundary of the CI was closer to zero than − 10% (Fig. 3).
Discussion
This study showed that the PPOS protocol with dydrogesterone was not inferior to the GnRH antagonist protocol for the primary outcome: the ongoing pregnancy rate. Similarly, the results of the PPOS protocol with dydrogesterone for one secondary outcome—the clinical pregnancy rate—also showed non-inferiority compared with those of the GnRH control group protocol. Several studies have already compared the PPOS protocol with the typical short GnRH agonist protocol and demonstrated that they are equivalent in efficacy and safety [5,6,7]. In this study, single-embryo FET was performed in most (90.8%) patients and the clinical and ongoing pregnancy rates were similar to the previous reports [5,6,7,8, 22]. Therefore, this study was considered a high-quality comparative study.
A premature LH surge can compromise the yield of oocytes and reduce the pregnancy rate [23]. The previous studies have shown that P4 when administered during the normal follicular phase reduces the LH pulse frequency, amplifies LH pulse amplitude, and reduces mean plasma LH levels compared with those in untreated women [24]. The previous studies in animal models have shown that P4 can block the E2-induced GnRH/LH surge-generating signal soon after the onset of signal transmission (immediately after E2 removal), but not during the later stages of signal transmission (at the time of onset of the surge) [25,26,27,28,29]. In addition, exogenous P4 administration timing is critical in determining whether it will stimulate or block the LH surge [25, 27, 28]. Zhu et al. reported that LH surge blockade failed if Utrogestan administration was started when the diameter of multiple follicles was > 10 mm [22]. Kuang et al. also showed that the medroxyprogesterone acetate and HMG protocol should be used when basal E2 levels are no greater than 50–70 pg/ml in cycles for avoiding a premature LH surge [5]. Therefore, we modified the regimen using dydrogesterone from days 2 to 3 of the menstrual cycle. We decided on a dosage of dydrogesterone at 20 mg per day, as reported by Kuang et al. [5, 30].
In the current study, pituitary LH levels on the trigger day were more strongly suppressed by daily oral dydrogesterone than by injection of a GnRH antagonist. This finding suggests that the hypothalamus and pituitary were more strongly suppressed in study group than in control group. This suppression might have resulted in a higher total HMG dose required, and earlier restarting of menstruation in study group than in control group.
No significant difference was found in the incidence of partial ovulation between study group (2/125) and control group (2/126). In all four cases of partial ovulation, the LH level on the triggering day did not rise, but leading follicle sizes were actually > 25 mm at retrieval. Partial ovulation can be caused by the sensitivity of ovaries and follicles to mechanical stimulation, such as puncturing, suggesting that partial ovulation was not associated with the protocol used.
Dydrogesterone is an established oral retroprogesterone that is approved for treatment of threatened and recurrent miscarriage, and infertility caused by luteal phase insufficiency. It has been used extensively for a variety of indications worldwide for an estimated 113 million women since 1960 (based on sales data). Approximately 20 million fetuses have been exposed to it in the uterus [14, 16, 31, 32]. This compound is structurally and pharmacologically similar to the natural endogenous P4. Moreover, it has greater affinity for P4 receptors than P4 itself. Therefore, it can be used in lower doses than P4 to promote endometrial proliferation thanks to its better bioavailability and to the P4-like activity of its metabolites [14]. Dydrogesterone also appears to have no affinity for androgen, estrogen, glucocorticoid, or mineralocorticoid receptors [15]. In Japan, dydrogesterone is popular as an oral medicine for luteal support. However, some reports have shown the efficacy of Utorogestan for preventing a premature LH surge during COH [6, 22, 33]. Utorogestan is approved only as a vaginal agent in Japan. This is why we chose oral dydrogesterone, which is a more convenient form than vaginal Utorogestan, for this PPOS protocol of COH.
Based on the limited available data, high P4 levels have no negative effect on follicular maturation, fertilization, and blastocyst formation [9, 10, 34]. Kuang et al. also reported that more than 500 children born from luteal-phase ovarian stimulation with high P4 levels in COH did not have an increased risk of congenital malformations compared with early-phase stimulation [9]. We need to confirm the long-term safety of ovarian stimulation using the dydrogesterone plus HMG protocol for the offspring of these women.
Using a GnRH agonist trigger for final maturation of oocytes is effective for preventing OHSS. In addition, the incidence of immature oocytes is reduced as a result of a GnRH agonist trigger [35]. This approach might enhance maturity of the oocyte nucleus and eventually increase the number of MII oocytes [36,37,38]. Therefore, we used a GnRH agonist trigger as our basic protocol.
Cost analysis for the medicines used in this study showed markedly lower costs for suppressing the premature LH surge in study group compared with control group. In study group, the overall cost of the LH surge suppression was around 13.1 United States Dollars (USD) compared with 189.6 USD in control group. Thus, study group achieved a 93.0% cost reduction compared with control group.
The dydrogesterone plus HMG protocol has the advantage of oral administration and user convenience; it is less painful and cheaper than GnRH antagonist protocols. However, a major limitation of this study is that the patients were not assigned to groups using randomization. We used alternating allocation to the two groups for this study, and this could not completely control for possible interfering factors. Therefore, fully validating this protocol for COH will need a randomized, controlled trial. In addition, a larger sample is required to confirm the feasibility of this new regimen and to evaluate the outcomes for children arising from it.
In conclusion, this prospective, controlled study showed that the dydrogesterone plus HMG protocol was not inferior to the GnRH antagonist protocol in terms of the ongoing pregnancy rate. The PPOS protocol using dydrogesterone may help to avoid a premature LH surge and decrease the incidence of OHSS while maintaining good pregnancy outcomes. Further research is required to confirm the feasibility of this regimen, such as the optimal dose of dydrogesterone, the method of triggering ovulation, and the long-term safety of progestin used for ART.
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- ART:
-
Assisted reproductive technology
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COCs:
-
Cumulus–oocyte complexes
- COH:
-
Controlled ovarian hyperstimulation
- E2:
-
Estradiol 2
- FET:
-
Frozen embryo transfer
- GnRH:
-
Gonadotropin-releasing hormone
- hCG:
-
Human chorionic gonadotropin
- HMG:
-
Human menopausal gonadotropin
- HRT:
-
Hormone replacement therapy
- IVF:
-
In vitro fertilization
- ICSI:
-
Intra-cytoplasmic sperm injection
- LH:
-
Luteinizing hormone
- OHSS:
-
Ovarian hyperstimulation syndrome
- P4:
-
Progesterone
- PPOS:
-
Progestin-primed ovarian stimulation
- SD:
-
Standard deviation
- USD:
-
United States dollar
References
Firouzabadi RD, Ahmadi S, Oskouian H, Davar R (2010) Comparing GnRH agonist long protocol and GnRH antagonist protocol in outcome the first cycle of ART. Arch Gynecol Obstet 281(1):81–85. https://doi.org/10.1007/s00404-009-1073-5
Mourad S, Brown J, Farquhar C (2017) Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev 1:CD012103. https://doi.org/10.1002/14651858.cd012103.pub2
Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ (2016) Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev 4:CD001750. https://doi.org/10.1002/14651858.cd001750.pub4
Reichman DE, Zakarin L, Chao K, Meyer L, Davis OK, Rosenwaks Z (2014) Diminished ovarian reserve is the predominant risk factor for gonadotropin-releasing hormone antagonist failure resulting in breakthrough luteinizing hormone surges in in vitro fertilization cycles. Fertility Steril 102(1):99–102. https://doi.org/10.1016/j.fertnstert.2014.04.010
Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, Ai A, Shoham Z (2015) Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertility Steril 104(1):62–70.e63. https://doi.org/10.1016/j.fertnstert.2015.03.022
Zhu X, Ye H, Fu Y (2016) The utrogestan and hMG protocol in patients with polycystic ovarian syndrome undergoing controlled ovarian hyperstimulation during IVF/ICSI treatments. Medicine (Baltimore) 95(28):e4193. https://doi.org/10.1097/md.0000000000004193
Wang Y, Chen Q, Wang N, Chen H, Lyu Q, Kuang Y (2016) Controlled ovarian stimulation using medroxyprogesterone acetate and hMG in patients with polycystic ovary syndrome treated for IVF: a double-blind randomized crossover clinical trial. Medicine (Baltimore) 95(9):e2939. https://doi.org/10.1097/MD.0000000000002939
Zhu X, Ye H, Fu Y (2017) Duphaston and human menopausal gonadotropin protocol in normally ovulatory women undergoing controlled ovarian hyperstimulation during in vitro fertilization/intracytoplasmic sperm injection treatments in combination with embryo cryopreservation. Fertility Steril 108(3):505–512. https://doi.org/10.1016/j.fertnstert.2017.06.017 (e502)
Kuang Y, Hong Q, Chen Q, Lyu Q, Ai A, Fu Y, Shoham Z (2014) Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen–thawed embryo transfer cycles. Fertility Steril 101(1):105–111. https://doi.org/10.1016/j.fertnstert.2013.09.007
Wang N, Wang Y, Chen Q, Dong J, Tian H, Fu Y, Ai A, Lyu Q, Kuang Y (2016) Luteal-phase ovarian stimulation vs conventional ovarian stimulation in patients with normal ovarian reserve treated for IVF: a large retrospective cohort study. Clin Endocrinol (Oxf) 84(5):720–728. https://doi.org/10.1111/cen.12983
Kwik M, Maxwell E (2016) Pathophysiology, treatment and prevention of ovarian hyperstimulation syndrome. Curr Opin Obstet Gynecol 28(4):236–241. https://doi.org/10.1097/gco.0000000000000284
Atkinson P, Koch J, Ledger WL (2014) GnRH agonist trigger and a freeze-all strategy to prevent ovarian hyperstimulation syndrome: a retrospective study of OHSS risk and pregnancy rates. Aust N Z J Obstet Gynaecol 54(6):581–585. https://doi.org/10.1111/ajo.12277
Yu S, Long H, Chang HY, Liu Y, Gao H, Zhu J, Quan X, Lyu Q, Kuang Y, Ai A (2018) New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: a randomized controlled trial including 516 first IVF/ICSI cycles. Hum Reprod 33(2):229–237. https://doi.org/10.1093/humrep/dex367
Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (2008) Classification and pharmacology of progestins. Maturitas 61(1–2):171–180
Schindler AE (2009) Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas 65(Suppl 1):S3–11. https://doi.org/10.1016/j.maturitas.2009.10.011
Nadarajah R, Rajesh H, Wong KY, Faisal F, Yu SL (2016) Live birth rates and safety profile using dydrogesterone for luteal phase support in assisted reproductive techniques. Singap Med J. https://doi.org/10.11622/smedj.2016080
Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF (1986) A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fertilization Embryo Transf 3(5):284–295
Toftager M, Bogstad J, Bryndorf T, Lossl K, Roskaer J, Holland T, Praetorius L, Zedeler A, Nilas L, Pinborg A (2016) Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod 31(6):1253–1264. https://doi.org/10.1093/humrep/dew051
Robert J (2009) Guidance for industry non-inferiority clinical trials. Food and Drug Administration, US Department of Health and Human Services Publishing online. https://www.fda.gov/downloads/drugs/drugsafety/informationbydrugclass/ucm187447.pdf. Accessed 31 July 2018
Althunian TA, de Boer A, Groenwold RHH, Klungel OH (2017) Defining the noninferiority margin and analysing noninferiority: an overview. Br J Clin Pharmacol 83(8):1636–1642. https://doi.org/10.1111/bcp.13280
Hahn S (2012) Understanding noninferiority trials. Korean. J Pediatr 55(11):403–407. https://doi.org/10.3345/kjp.2012.55.11.403
Zhu X, Zhang X, Fu Y (2015) Utrogestan as an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Medicine (Baltimore) 94(21):e909. https://doi.org/10.1097/MD.0000000000000909
Messinis IE (2006) Ovarian feedback, mechanism of action and possible clinical implications. Hum Reprod Update 12(5):557–571. https://doi.org/10.1093/humupd/dml020
Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ (1984) Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab 58(2):378–383. https://doi.org/10.1210/jcem-58-2-378
Harris TG, Dye S, Robinson JE, Skinner DC, Evans NP (1999) Progesterone can block transmission of the estradiol-induced signal for luteinizing hormone surge generation during a specific period of time immediately after activation of the gonadotropin-releasing hormone surge-generating system. Endocrinology 140(2):827–834. https://doi.org/10.1210/endo.140.2.6490
Richter TA, Robinson JE, Evans NP (2002) Progesterone blocks the estradiol-stimulated luteinizing hormone surge by disrupting activation in response to a stimulatory estradiol signal in the ewe. Biol Reprod 67(1):119–125
Richter TA, Robinson JE, Lozano JM, Evans NP (2005) Progesterone can block the preovulatory gonadotropin-releasing hormone/luteinising hormone surge in the ewe by a direct inhibitory action on oestradiol-responsive cells within the hypothalamus. J Neuroendocrinol 17(3):161–169. https://doi.org/10.1111/j.1365-2826.2005.01287.x
Pohl CR, Richardson DW, Marshall G, Knobil E (1982) Mode of action of progesterone in the blockade of gonadotropin surges in the rhesus monkey. Endocrinology 110(4):1454–1455. https://doi.org/10.1210/endo-110-4-1454
Dierschke DJ, Yamaji T, Karsch FJ, Weick RF, Weiss G, Knobil E (1973) Blockade by progesterone of estrogen-induced LH and FSH release in the rhesus monkey. Endocrinology 92(5):1496–1501. https://doi.org/10.1210/endo-92-5-1496
Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (2003) Classification and pharmacology of progestins. Maturitas 46(Suppl 1):S7–S16
Schindler AE (2016) Present and future aspects of dydrogesterone in prevention or treatment of pregnancy disorders: an outlook. Horm Mol Biol Clin Investig 27(2):49–53. https://doi.org/10.1515/hmbci-2016-0028
Tournaye H, Sukhikh GT, Kahler E, Griesinger G (2017) A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod 32(5):1019–1027. https://doi.org/10.1093/humrep/dex023
Zhu X, Ye H, Fu Y (2017) Use of utrogestan during controlled ovarian hyperstimulation in normally ovulating women undergoing in vitro fertilization or intracytoplasmic sperm injection treatments in combination with a “freeze all” strategy: a randomized controlled dose-finding study of 100 mg versus 200 mg. Fertility Steril 107(2):379–386. https://doi.org/10.1016/j.fertnstert.2016.10.030 (e374)
Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, Trabucco E, Venturella R, Vajta G, Rienzi L (2016) Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertility Steril 105(6):1488–1495.e1481. https://doi.org/10.1016/j.fertnstert.2016.03.002
Griffin D, Benadiva C, Kummer N, Budinetz T, Nulsen J, Engmann L (2012) Dual trigger of oocyte maturation with gonadotropin-releasing hormone agonist and low-dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertility Steril 97(6):1316–1320. https://doi.org/10.1016/j.fertnstert.2012.03.015
Zelinski-Wooten MB, Hutchison JS, Hess DL, Wolf DP, Stouffer RL (1995) Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys. Hum Reprod 10(7):1658–1666
Imoedemhe DA, Sigue AB, Pacpaco EL, Olazo AB (1991) Stimulation of endogenous surge of luteinizing hormone with gonadotropin-releasing hormone analog after ovarian stimulation for in vitro fertilization. Fertility Steril 55(2):328–332
Humaidan P, Bredkjaer HE, Bungum L, Bungum M, Grondahl ML, Westergaard L, Andersen CY (2005) GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod 20(5):1213–1220. https://doi.org/10.1093/humrep/deh765
Acknowledgements
The authors wish to thank Ms. Mika Matsuoka for data collection, and Ms. Nami Hirayama and Ms. Yumiko Kobayashi for statistical analysis (clinical staff in the Kamiya Ladies Clinic). We also thank Dr. Shigeo Araki (Chief Director of the International Institute of Medical Technology IMT College) and Dr. Daiki Iwami (staff member of the Department of Renal and Genitourinary Surgery, Hokkaido University, Graduate School of Medicine) for proofreading the manuscript, and Dr. Kota Ono (staff member of the Department of Biostatistics, Hokkaido University, Graduate School of Medicine) as a statistical adviser. We thank Ellen Knapp, PhD, and James Cummins, PhD, from Edanz Group (http://www.edanzediting.com/ac) for editing drafts of this manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
NI: Protocol development, data analysis, data collection, manuscript writing. MK: Data collection. NO: Data collection. TY: Data collection. EW: Data collection. OM: Data collection. HK: Data collection, protocol development.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the Kamiya Ladies Clinic and with the 1964 Helsinki declaration and its later amendments or similar ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Iwami, N., Kawamata, M., Ozawa, N. et al. New trial of progestin-primed ovarian stimulation using dydrogesterone versus a typical GnRH antagonist regimen in assisted reproductive technology. Arch Gynecol Obstet 298, 663–671 (2018). https://doi.org/10.1007/s00404-018-4856-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4856-8