Abstract
Objective
To evaluate the effect of Diane-35, alone or in combination with orlistat or metformin, on androgen and body fat percentage parameters in Chinese overweight and obese polycystic ovary syndrome (PCOS) patients with insulin resistance.
Methods
A total of 240 PCOS women were randomly allocated to receive Diane-35 alone (D group), Diane-35 plus orlistat (DO group), Diane-35 plus metformin (DM group), or Diane-35 plus orlistat plus metformin (DOM group). Serum TT, DHEA-S, androstenedione, SHBG, FT, FAI, body fat, and body fat percentage were assessed at baseline and after 12 weeks of treatment.
Results
Significant changes in serum TT, SHBG, and FAI were observed in all treatment groups compared with baseline. DHEA-S and androstenedione significantly decreased in the DO, DM, and DOM groups after treatment. FT only significantly decreased in the DOM group. Body fat and body fat percentage significantly decreased in the DO and DOM groups. Compared with the D group, DHEA-S significantly decreased in the DO, DM, and DOM groups (F = 4.081, p = 0.008); SHBG significantly increased in the DOM group (F = 3.019, p = 0.031); and FAI significantly decreased in the DO group (χ2 = 12.578, p = 0.006). There were significant differences between groups in body fat percentage (χ2 = 23.590, p < 0.001). Side-effects were less with orlistat than metformin.
Conclusions
Diane-35 in combination with orlistat or metformin is more effective in reducing androgen than Diane-35 alone. Orlistat is more effective in reducing body fat percentage than metformin. In addition, orlistat has mild side-effects and is better tolerated compared with metformin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder of reproductive-aged women around the world [1]. PCOS affects 6–21% of the female population [2]. The prevalence of PCOS depends on ethnicity, environmental, and genetic factors [3]. Clinical presentation can vary widely but, in general, PCOS is characterized by hyperandrogenism, polycystic ovaries, and anovulation, after excluding other endocrine causes such as hyperprolactinemia [4]. The majority of women with PCOS have insulin resistance and/or are obese [5]. The risk of metabolic and cardiovascular disease and gynecological cancers associated with PCOS highlight the importance of early diagnosis and appropriate multidisciplinary management. Lifestyle modification, including diet, exercise, and behavioral modification, is the first line in the management of all women with PCOS [6].
Pharmacological treatment of PCOS includes hormonal contraceptives, metformin, myo-inositol, orlistat, and GLP1 agonists. Diane-35 contains 2-mg cyproterone acetate (CPA) and 35-μg ethinylestradiol (EE), which is the first choice for the management of PCOS patients with hyperandrogenism in China [7]. The antiandrogenic effect of Diane-35 is achieved via many different mechanisms. Diane-35 can reduce biochemical hyperandrogenism and can treat clinical signs of hyperandrogenism such as acne, seborrhea, and hirsutism, and is also active as an oral contraceptive [8]. Metformin is a first-line medication for metabolic manifestations such as hyperglycemia [9]. Metformin works by inhibiting the production of hepatic glucose, reducing intestinal glucose absorption and improving glucose uptake and utilization [10]. Besides lowering the blood glucose level, metformin may have additional health benefits including weight reduction, lowering plasma lipid levels, and prevention of some vascular complications [11]. Orlistat, an FDA-approved drug, is an effective medication in the treatment of obesity. Orlistat is a potent and irreversible inhibitor which prevents the hydrolysis of dietary fat into absorbable-free fatty acids and monoglyceride with an increase in fecal fat excretion [12, 13]. Orlistat has excellent effects on reducing body weight and body mass index in overweight and obese patients [14]. Previous studies which have compared the effects of Diane-35 alone or in combination with metformin in PCOS patients showed favorable results [15, 16]. However, they only investigated a small number of cases and were not always well randomized. The aim of this study was to evaluate the effect of Diane-35, alone or in combination with orlistat or metformin on androgen and body fat percentage parameters in Chinese overweight and obese PCOS patients within a prospective randomized study.
Methods
Participants
The study was approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All participants gave their informed consent before enrolling in the study. Consecutive patients attending the Department of Gynecological Endocrinology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University (Beijing, China), were recruited between July 2016 and April 2017. Women were included in the study if they met all the following conditions: being Chinese, diagnosis of PCOS, aged between 18 and 40 years, body mass index (BMI) ≥ 24 kg/m2, fasting insulin (FINS) > 10 mIU/L, and no history of taking medication or dietary modification currently or for the preceding 3 months. The diagnosis of PCOS was made according to the Rotterdam criteria with the presence of at least two of the following three features: oligo- or anovulation, clinical and/or biochemical hyperandrogenism and ultrasound finding of polycystic ovaries (presence of 12 or more follicles in each ovary measuring 2–9 mm in diameter, and/or increased ovarian volume > 10 ml), and exclusion of other etiologies (congenital adrenal hyperplasia, Cushing’s syndrome, androgen-secreting neoplasms, hyperprolactinemia, and thyroid disease). Exclusion criteria were ischemic heart disease, clinically evident vascular disease, type-2 diabetes with ketoacidosis, renal or hepatic impairment, severe infection, and malignant tumor.
Study design
The trial was a randomized, open-label, and parallel study. A total of 240 PCOS patients were randomly divided into four groups: (1) Diane-35 alone (D group); (2) Diane-35 + Orlistat (DO group); (3) Diane-35 + metformin (DM group); and (4) Diane-35 + Orlistat + metformin (DOM group). Randomization was performed using a random number table. The four groups of patients were treated for 12 weeks. At baseline, a dietician prescribed an individualized low-fat diet and moderate daily physical activity, based on each patient’s basal energy requirements and on an estimation of the typical activity level. Diane-35 was taken once daily at the same time from the 5th day of menstruation or withdrawal bleeding for a period of 21 days and for 3 menstrual cycles. The dose of orlistat was 120 mg three times daily before each meal, and the dose remained constant throughout the study period. The dose of metformin was increased stepwise, from 500 mg once daily for the first week, to 500 mg twice daily for the next week, to 500 mg three times daily for the remaining study period. The subjects attended two interim checks once every month for a compliance check and to record any side effects.
Study measurements
Clinical and biochemical assessments were performed at baseline and at the end of the 12-week period. The body weight, height, waist circumference, body fat, and body fat percentage were measured after an overnight fast with subjects wearing light clothes and no shoes. Blood samples were taken between the second and fourth day of a spontaneous menstrual cycle or at any time point in the menstrual cycle with no follicle of a diameter greater than 10 mm by vaginal ultrasonography after an overnight fast. The androgen profile including serum total testosterone (TT), dehydroepiandrosterone sulfate (DHEA-S), androstenedione, sex hormone-binding globulin (SHBG), free testosterone (FT), and free androgen index (FAI) were measured using an ADVIA Centaur XP immunoassay system (Siemens, Germany). Body fat and body fat percentage were measured using an MC/MES00-042 muscle function analyzer (Maidakang, China).
Statistical analysis
All statistical analyses were performed using SPSS 22.0 for Windows (IBM SPSS Statistics, IBM software, Armonk, NY). Values were given as mean ± standard deviation (SD) or median (interquartile range). Continuous variables were tested for normality by the Kolmogorov–Smirnov test. The analysis of the differences between groups was assessed by the ANOVA or the Kruskal–Wallis test for parametric or non-parametric data, respectively. The paired-samples’ t test or the paired Wilcoxon test was used for assessing the effect of treatment for parametric or non-parametric data, respectively. Significance was defined as p < 0.05.
Results
All 240 included subjects completed the 12-week study period. The baseline demographic and clinical characteristics were similar in all four groups with no significant differences (Table 1).
Androgen parameters
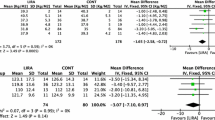
When compared with baseline, we observed significant reductions in serum TT and FAI, with significant increases in SHBG in all treatment groups. DHEA-S and androstenedione were significantly reduced in the DO (p < 0.001 and p = 0.026, respectively), DM (p < 0.001 and p < 0.001, respectively), and DOM (p < 0.001 and p = 0.001, respectively) groups. FT was significantly reduced in the DOM (p = 0.002) group. Compared with the D group, DHEA-S reductions were significantly greater in the DO, DM, and DOM groups (F = 4.081, p = 0.008); SHBG levels were significantly increased in the DOM group (F = 3.019, p = 0.031); and the reductions in FAI were significantly greater in the DO group (χ2 = 12.578, p = 0.006) (Table 2).
Body fat and body fat percentage features
At the end of the 12-week treatment period, significant reductions in body fat and body fat percentage were observed in the DO (p = 0.005 and p = 0.002, respectively) and DOM (p < 0.001 and p < 0.001, respectively) groups (Figs. 1, 2). There were significant differences between groups in body fat percentage (χ2 = 23.590, p < 0.001). The reductions were significantly greater in the DO group compared with the D group, while the reductions were significantly greater in the DOM group compared with the D and DM groups.
Safety
Twenty-one patients who took Diane-35 experienced side effects including headaches, nausea, weight gain, breast tenderness, and loss of libido. However, in general, Diane-35 was well tolerated and we did not observe any cases of venous thrombosis. Five patients who received orlistat experienced gastrointestinal adverse effects (mainly flatulence and oily spotting) during the first 2 weeks of treatment, which decreased in frequency with ongoing treatment. No subject stopped treatment or required any dose reduction. Nine patients taking metformin 1500 mg/day reported side-effects such as mild nausea and abdominal pain. Four patients tolerated the dose gradually, while in another five patients the tolerated dose was 1000 mg/day.
Discussion
In this open-label randomized study, we examined the effect of Diane-35, alone or in combination with orlistat or metformin, in Chinese overweight and obese PCOS patients with insulin resistance. Obesity is a major risk factor of PCOS and is usually of the central type [17]. Obesity exacerbates hormonal and clinical features of PCOS, and women with PCOS appear to be at higher risk of obesity, with multiple underlying mechanisms [18]. Insulin resistance appears to be the fundamental key factor within the pathophysiology of PCOS [19, 20]. Diane-35 is a reliable drug for women who suffer from PCOS. It works by blocking the effects of androgens such as testosterone and by activating the progesterone receptor. In addition, it increases serum SHBG synthesized in the liver, thus decreasing free T. Orlistat is a drug which can induce weight loss with minimal systemic absorption. Therefore, any effect is a result of weight loss and not of any direct effect on ovaries [21]. Metformin has an insulin-lowering effect by improving insulin sensitivity and in turn, can decrease circulating androgen levels. In our study, significant reductions in serum total testosterone and FAI were observed in all treatment groups after 12 weeks of treatment. SHBG synthesis in the liver is down-regulated by hyperinsulinemia and has increased androgen levels [21]. Therefore, a significant increase in SHBG was observed in all the treatment groups. Similar effects of Diane-35 or Diane-35 plus metformin on TT in PCOS patients were observed in an earlier study [22]. A previous study also demonstrated the same effect of orlistat in women with PCOS [23]. Combination treatment appeared to be more effective in reducing androgen compared with monotherapy.
Weight gain is often considered to be a side effect of hormonal contraceptives. Concern about weight gain can limit the use of the medication in terms of initiation or cause early discontinuation among users. However, available evidence is insufficient to determine the effect of contraceptives on weight, since no large effect is evident [24]. In our study, we observed reductions in body fat and body fat percentage in all treatment groups, with significant reductions in the DO and DOM groups. There were also reductions in the D and DM groups, but without significant differences (p>0.05). A previous study reported that metformin alone or in combination with OCPs was associated with weight loss and improved body composition compared with OCP alone [25]. Our study indicated that Diane-35 plus orlistat is better for body composition than Diane-35 alone or in combination with metformin. Orlistat is more effective in reducing body fat and body fat percentage than metformin.
Combined oral contraceptives have been found to be associated with an increased risk of venous thrombosis, depending on the estrogen dose and type of progestogen [26, 27]. In addition, two observational studies suggested that the risk of venous thrombosis is elevated in PCOS itself [28, 29]. In our study, no patient suffered a venous thrombosis during 12 weeks of treatment. More studies and especially prospective, well-designed trials are needed to assess the risk of venous thrombosis in PCOS patients with Diane -35.
There could be some limitations to our study: in China, Diane-35 is the main drug used to treat polycystic ovary syndrome. Therefore, all patients in our study were treated with Diane-35. However, since we compared combinations versus Diane-35 alone in a randomized study design, we were able to evaluate possible effects of orlistat and metformin, respectively. Another limitation may be that the treatment duration of 12 weeks might be too short to assess all possible effects. However, regarding effects on androgen parameters, relevant differences should be seen when comparing our results with other studies, although we cannot exclude changes in these effects during longer treatment durations.
In conclusion, Diane-35 in combination with orlistat or metformin appears to be an effective and safe treatment option for overweight and obese PCOS patients. Combination treatment is more effective in reducing androgens than Diane-35 alone. Orlistat is more effective in reducing body fat and body fat percentage than metformin. In addition, orlistat has mild side effects and is better tolerated compared to metformin. However, long-term, double-blind, placebo-controlled studies are needed to confirm our findings.
References
Goodman NF, Cobin RH, Futerweit W et al (2015) American association of clinical endocrinologists, American college of endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—PART 2. Endoc Pract 21:1415–1426
Lizneva D, Suturina L, Walker W et al (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106:6–15
Ali AT (2015) Polycystic ovary syndrome and metabolic syndrome. Ceska Gynekol 80:279–289
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25
Royal College of Obstetricians and Gynecologists (2014) Polycystic ovary syndrome, long-term consequences (Green-top Guideline No. 33). https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg33/. Accessed 05 Nov 2014
Conway G, Dewailly D, Diamanti-Kandarakis E et al (2014) The polycystic ovary syndrome: a position statement from the European society of endocrinology. Eur J Endocrinol 171:1–29
Buzney E, Sheu J, Buzney E et al (2014) Polycystic ovary syndrome: a review for dermatologists, Part II. Treatment. J Am Acad Dermatol 71:859.e1–859.e15
Ruan X, Kubba A, Aguilar A et al (2017) Use of cyproterone acetate/ethinylestradiol in polycystic ovary syndrome: rationale and practical aspects. Eur J Contracept Reprod Health Care 22:183–190
Williams T, Mortada R, Porter S (2016) Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician 94:106–113
Wang YW, He SJ, Feng X et al (2017) Metformin: a review of its potential indications. Drug Des Devel Ther 11:2421–2429
Ladson G, Dodson WC, Sweet SD et al (2011) The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril 95:1059–1066
Kumar P, Arora S (2014) Orlistat in polycystic ovarian syndrome reduces weight with improvement in lipid profile and pregnancy rates. J Hum Reprod Sci 7:255–261
Alqahtani S, Qosa H, Primeaux B et al (2015) Orlistat limits cholesterol intestinal absorption by Niemann-pick C1-like 1 (NPC1L1) inhibition. Eur J Pharmacol 762:263–269
Ahn SM, Kim H, Ji E et al (2014) The effect of orlistat on weight reduction in obese and overweight Korean patients. Arch Pharm Res 37:512–519
Wu H, Ruan X, Jin J et al (2015) Metabolic profile of Diane-35 versus Diane-35 plus metformin in Chinese PCOS women under standardized life-style changes. Gynecol Endocrinol 31:548–551
Yang YM, Choi EJ (2015) Efficacy and safety of metformin or oral contraceptives, or both in polycystic ovary syndrome. Ther Cin Risk Manag 11:1345–1353
Vilmann LS, Thisted E, Baker JL et al (2012) Development of obesity and polycystic ovary syndrome in adolescents. Horm Res Paediatr 78:269–278
Naderpoor N, Shorakae S, Joham A et al (2015) Obesity and polycystic ovary syndrome. Minerva Endocrinol 40:37–51
Baptiste CG, Battista MC, Trottier A et al (2010) Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol 122:42–52
Tosi F, Bonora E, Moghetti P (2017) Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum Reprod 13:1–7
Nestler JE, Powers LP, Matt DW et al (1991) A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 72:83–89
Feng W, Jia YY, Zhang DY et al (2016) Management of polycystic ovarian syndrome with Diane-35 or Diane-35 plus metformin. Gynecol Endocrinol 32:147–150
Panidis D, Tziomalos K, Papadakis E et al (2014) The role of orlistat in combination with lifestyle changes in the management of overweight and obese patients with polycystic ovary syndrome. Clin Endocrinol (Oxf) 80:432–438
Gallo MF, Lopez LM, Grimes DA et al (2014) Combination contraceptives: effects on weight. Cochrane Database Syst Rev 29:CD003987
Glintborg D, Altinok ML, Mumm H et al (2014) Body composition is improved during 12 months’ treatment with metformin alone or combined with oral contraceptives compared with treatment with oral contraceptives in polycystic ovary syndrome. J Clin Endocrinol Metab 99:2584–2591
de Bastos M, Stegeman BH, Rosendaal FR et al (2014) Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev 3:CD010813
Sandset PM (2014) Combined oral contraceptives increase risk of venous thrombosis according to oestrogen dose and type of progestogen. Evid Based Med 19:194
Okoroh EM, Hooper C, Atrash HK et al (2012) Is polycystic ovary syndrome anther risk factor for venous thromboembolism? United States 2003–2008. Am J Obstet Gynecol 207(377):e1–e8
Bird ST, Hartzema AG, Brophy JM et al (2013) Risk of venous thromboembolism in women with polycystic ovary syndrome: a population-based matched cohort analysis. CMAJ 185:E115–E120
Acknowledgements
The authors especially thank Prof. Xingming Li for his assistance in statistical analysis of the data.
Author information
Authors and Affiliations
Contributions
XR: Project development, manuscript writing; JS: data collection, data analysis; MG: data collection; LW: data collection; HW: data collection; AOM: manuscript editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by Capital Characteristic Clinic Project of China (Z161100000516143); Beijing Capital Foundation for Medical Science Development and Research (2016-2-2113); Clinical Technique Innovation Project of Beijing Municipal Administration of Hospitals (XMLX201710); Beijing Municipality Health Technology High-level Talent (2014-2-016); Foreign technical and administrative talent introduction project in 2017, State Administration of Foreign Experts Affairs, the P. R. of China (20171100004).
Rights and permissions
About this article
Cite this article
Ruan, X., Song, J., Gu, M. et al. Effect of Diane-35, alone or in combination with orlistat or metformin in Chinese polycystic ovary syndrome patients. Arch Gynecol Obstet 297, 1557–1563 (2018). https://doi.org/10.1007/s00404-018-4762-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4762-0