Abstract
Purpose
To assess whether there are proteins in endometrial fluid aspirate (EFA) that predict implantation.
Methods
The population under study consisted of 285 women undergoing embryo transfer (ET). Endometrial fluid aspiration was performed immediately before ET. Results of proteomic analysis of EFA were compared between 33 cases who achieved pregnancy and 33 who did not. Samples were analysed by 2D electrophoresis and mass spectrometry. Blood samples were studied by ELISA Pregnancy rates and maternal complications were compared to those in women refusing aspiration.
Results
We found 23 proteins differentially expressed in the EFA in conception cycles: 4 up-regulated proteins and 19 down-regulated (FC = 0.31 0.78) (among others, arginase-1, actin B, PARK-7, cofilin-1, stathmin, annexin-2 and CAPZB). Among the five studied proteins that were differentially expressed in EFA, none was differentially expressed in serum. The aspiration procedure had no impact on pregnancy rate. No maternal complications were reported.
Conclusions
We found a very different protein profile in implantative cycles, the majority of proteins being down-regulated. This probably reflects a different endometrial functional status, more favourable to implantation. EFA proteomic analysis could be a useful tool in the planning ET strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embryo implantation is one of the most inefficient steps in assisted reproduction techniques [1]. Currently, endometrial ultrasound is the only universally accepted tool to study the influence of the endometrium on implantation [2].Endometrial tissues have been studied by histological, histochemical, and biochemical methods in the last two decades. A large number of proteins and other molecules, which are expressed in the endometrium in a cycle-dependent manner, have been described [3]. However, most techniques used to investigate endometrial receptivity require an endometrial biopsy, which precludes their use in the same cycle as embryo transfer (ET).
In recent years, some works have been directed to the analysis of uterine cavity lavage samples [4] or even directly endometrial fluid with no lavage [5, 6]. Uterine fluid is a protein-rich histotroph that contains secretions from the endometrial glands and cleavage products of both the secreted proteins and the glycocalyx (the glycoprotein mucin-rich layer coating the endometrial apical cell surface) [7]. Glandular secretions are known to be essential for implantation in sheep [8] and mice [9].
Our research group has developed a non-invasive technique for analysing endometrial proteins in endometrial fluid aspirate (EFA) obtained during the window of implantation. We have previously reported that more than 800 proteins can be detected in this fluid by proteomic techniques [5].
Most previous studies on endometrial markers have been performed using genomic or proteomic techniques in endometrial cells [10, 11]. Only a few used proteomic or genomic approaches to analyse endometrial cavity lavage fluid [4], and as far as we know, only one focused on endometrial fluid by lipidomics [12]. Most researchers take as the gold standard the “receptive endometrium”, that is, the endometrium when it is developmentally competent for implantation [7]. It is not clear, however, whether (1) a receptive endometrium, when receiving a good quality embryo, always produces a pregnancy; or (2) the timing of the endometrium being receptive is the same in different women or in different cycles of the same woman. In addition, controlled ovarian stimulation used in IVF cycles considerably alters the endometrium [13, 14], resulting in both inadequate receptivity and/or changes in its timing. In our opinion, the gold standard for studying the endometrium, from a reproductive point of view, should be the “implantative” endometrium, that is, the endometrium where implantation occurs in the very same cycle.
The hypothesis of the present study is that, in IVF cycles, a different level of endometrial development might yield a different protein secretion pattern, and that the implantation outcome might be associated with some of these proteins. The knowledge of such patterns could be of great interest allowing different alternatives: in poor prognosis cases, cancellation of the ET (freezing oocytes or embryos) or even increasing the number of embryos to be transferred, and in good prognosis, reducing the number of embryos transferred. The second part of our study was focused on ascertaining whether the protein markers of implantation detected in the EFA could also be detected and validated in a paired blood sample also obtained at the time of ET.
Materials and methods
The population under study consisted of 285 women undergoing IVF at the Reproductive Unit of Cruces University Hospital (University of the Basque Country).
The inclusion criteria were: (i) age under 40 years, (ii) fresh ET, (iii) no more than two previous IVF cycles, (iv) ET performed on day 2–3, (v) absence of polyps, myoma, and hydrosalpinx, (vi) absence of infectious risk (no history of pelvic inflammatory disease, endometritis, endometrioma, HIV or sexually transmitted diseases), (vii) no requirement for oocyte donation, preimplantation genetic diagnosis or testicular biopsy, and (viii) easy previous mock transfer. We obtained approval from the Institutional Review Board (CEIC 09/54 and CEIC 11/45) and informed consent from participants.
In the 285 women recruited, endometrial fluid was aspirated using the catheter used for ET (Frydman, Instrumentos Médicos Estériles SA, Spain) connected to a 10 mL syringe, just prior to ET, under abdominal ultrasound guidance. Sample extraction was performed by gentle manual application of negative pressure with the syringe. To prevent contamination with cervical mucus, aspiration was interrupted at the internal cervical os. Special care was taken in the collection procedure to avoid touching the uterine fundus or injuring the cervix, and to minimize sample contamination with blood and endometrial tissue. In cases with excessive vaginal secretions, the vagina was cleaned with saline solution. Aspirated samples were expelled into standard cryogenic tubes and immediately frozen at − 80 °C until processed. Aspirate volumes ranged from 5 up to 50 µL. Such range is consistent with previously reported data [6]. 5 min after ET, a blood sample was taken by venipuncture (10 mL) and serum was obtained by routine centrifugation. Serum samples were also frozen (− 80 °C) until processing.
Of the 285 EFAs obtained, 35 were discarded due to insufficient sample volume [10] or visually evident blood contamination [25]. From the remaining 250, the first consecutive 33 samples corresponding to ET resulting in pregnancy were selected for proteomic analysis, while the control group was composed of 33 samples from women in whom ET did not result in pregnancy, immediately following each pregnancy case. Biochemical pregnancies and ectopic pregnancies were excluded. The remaining 194 samples have not yet been analysed.
For the safety analysis, we also included 200 oocyte donors who underwent conventional ovarian stimulation, from whom EFA was obtained on day 3 after oocyte pick-up.
Ovarian cycle management in our IVF patients has been described previously. It consisted in either a long agonist protocol or a conventional antagonist protocol. Ovarian stimulation was performed with recombinant (rec) FSH (in women ≤ 35 years), and with rec FSH plus hMG or with rec FSH and rec LH (in women aged 36–39). Rec hCG was given s.c. at a dose of 250 mcg when at least three follicles were observed to have reached a mean diameter of 18.5 mm. Transvaginal ultrasound-guided follicular aspiration was scheduled 36 h after hCG injection [1]. The oocyte donor protocol consisted of recombinant FSH with antagonist short protocol and triggering with 0.2 mg of triptorelin s.c.
At the moment of the study, the ET policy consisted of transferring, when available, two embryos in good prognosis cases (woman age < 37 years, good quality embryos) and three embryos in poor prognosis cases (woman age ≥ 38 years, poor quality embryos, third IVF cycle).
Phases of the study
Our study was divided into four different phases: (1) preliminary safety analysis; (2) EFA analysis; (3) serum sample analysis; and (4) overall safety analysis.
Preliminary safety phase
This safety study (approved by the Institutional Board Review, ref. CEIC 09/54) was designed as a non-inferiority trial. The purpose was to analyse 30 cases and compare them to matched controls (considering age, infertility diagnosis, ovarian stimulation protocol, estradiol level, and numbers of oocytes obtained, top-quality embryos, and embryos transferred) treated over the same period of time.
EFA sampling was to be considered safe if the pregnancy rate (PR) was the same or higher in the study group. If the PR were ≥ 10% lower in the study group, the aspiration would be considered unsafe and the study would be halted. If the PR were between 0.1 and 9.9% lower, a further 30 cases would be studied, and the study would be halted if the total PR were > 1% lower.
Once the non-inferiority had been demonstrated in the preliminary safety study, the investigational study was undertaken. The investigational study was approved by our Institutional Review Board (code CEIC 11/45). Informed consent was obtained from all participating women (regardless of whether their data were used for the safety or the investigational phase).
EFA analysis
Protein extraction
Protein extraction was done as described previously [6], and protein content was determined using Bio-Rad Protein Assays (Bio-Rad) following the manufacturer’s instructions.
2D electrophoresis analysis
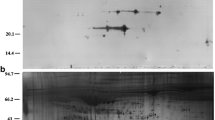
First dimension electrophoresis was performed on immobilized pH gradient strips (pH 3–10) using an Ettan™ IPGphor™ 3 System (GE Healthcare) and second dimension by SDS-PAGE was performed in an Ettan™ DALT Twelve Gel Caster (GE Healthcare) as described previously [6]. 2D gels were stained using Flamingo Fluorescent gel stain (Bio-Rad), and scanned on a Typhoon Trio scanner (GE Healthcare) for subsequent image analysis of the protein spots. For some samples, more than one 2D gel was run. A representative 2D image obtained from an EFA sample is shown in Fig. 1.
Analysis of the protein spots
Digitalised 2D proteomes were analysed using the Progenesis PG240 version 2007 software (Nonlinear Dynamics, Newcastle upon Tyne, UK) [6].
Statistical analysis
Statistical analysis was performed using IBM SPSS (IBM, Armonk, NY,USA). Differences in each of the biomarkers between the groups achieving and not achieving pregnancy were assessed with the Mann–Whitney U test. A p value of 0.05 was considered the threshold for statistical significance. The fold change (FC) in the expression level of each protein between the two groups was obtained by comparing the medians of the intensity expression values obtained from the Progenesis software. The differential spots were checked on the 2D images to rule out matching errors and when necessary, the matching was corrected and the statistical analysis was repeated.
Pregnancy was defined as the visualization of a gestational sac 4 weeks after embryo transfer.
Protein identification
A preparative 2D gel was run and stained using silver nitrate (Silver Staining Kit, ref 17-1150-01, GE Healthcare). Differentially expressed protein bands were trypsinized and resulting peptides were analysed by a combination of peptide mass and peptide fragment fingerprinting in an Autoflex Smartbeam System (Bruker Daltonics), using the Mascot search engine (Matrix Science) against Uniprot database.
Serum analysis: validation of biomarkers
Serum was extracted from blood samples by centrifugation (10 min, 3000 rpm). Five proteins selected from the mass spectrometry analysis were quantitatively detected in serum using commercial enzyme-linked immunosorbent assay (ELISA) tests following the manufacturer’s instructions: annexin A2 (ANXA2), capping protein (actin filament) muscle Z-line, beta (CAPZB), cofilin 1 (non-muscle) (CFL1), Parkinson protein 7 (PARK7) and stathmin 1 (STMN1) (catalogue numbers:ABIN1113424, ABIN829266and ABIN1568789, ABIN1114233, ABIN366580ABIN1874419and ABIN1117229, respectively, antibodies-online GmbH, Germany).
Global safety phase
The pregnancy rate obtained among the 285 women who underwent endometrial fluid aspiration was compared to a matched population (considering age, infertility diagnosis, ovarian stimulation protocol, estradiol level, and the numbers of oocytes obtained, top-quality embryos, and embryos transferred) treated over the same period of time who declined to participate in the study. The rates of infection and of haemorrhage after ET were also compared between the two aforementioned populations. Infection/haemorrhage rates were also studied among the 200 oocyte donors who had also undergone endometrial fluid aspiration.
Results
Pre-study safety analysis
The pregnancy rate among the studied 30 patients was unaffected by the collection of the endometrial fluid sample when compared to the control group of patients of similar characteristics assisted during the same period of time. Pregnancy rates were similar in the two groups: 40.0% in the study group (12/30) vs. 36.7% (11/30) in the control group. There were no significant differences between both groups in regards to demographic and clinical parameters (Table 1).
EFA analysis
Table 2 lists the proteins for which the spot intensity was significantly different (p < 0.05), proteins subsequently being identified by mass spectrometry. Each spot number is represented along with the corresponding protein acronym and Uniprot code. The FC in expression level of each protein between the groups studied is also included. FC > 1 indicate that the protein was overexpressed and FC < 1 that the protein was down-regulated. In some cases, the same protein was identified in two or more different spots, corresponding to different isoforms or post-translational modifications of the same protein.
Of the approximately 800 observed spots in the 2D gels, we found 23 proteins that were differentially expressed among women achieving and not achieving pregnancy. Most of the proteins were down-regulated (n = 19), with FC ranging from 0.31 to 0.78. Just four proteins were up-regulated: catalase, serum albumin, serotransferrin and Ig kappa chain V, with FC ranging from 1.43 to 1.92. The last three should be considered as having a blood origin or non-specific source.
Serum analysis
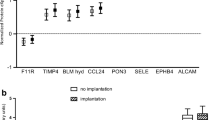
ELISA analysis of blood samples obtained 5 min after collection of endometrial fluid was used to study five proteins: cofilin-1; stathmin, annexin-2, CAPZβ, and PARK7. No significant differences in any of these five proteins were found between conception and non-conception cycles (Fig. 2). The ELISA kits used failed to detect CAPZβ.
Comparison of the serum levels of four proteins significantly different in endometrial fluid aspirate (conception vs. no conception cycles). None of the differences were significant. The upper horizontal line of the box corresponds to the 75th percentile (Q3) and the lower horizontal line of box to the 25th percentile (Q1); the horizontal bar within box is the median. The upper horizontal bar outside box, is calculated by this expression: Q3 + 1.5(Q3 − Q1) and the lower horizontal bar outside box: Q1 − 1.5(Q3 − Q1). Circles represent outliers. CFL-1 cofilin 1, PARK7 Protein DJ-1, ANXA2 annexin 2
EFA and serum sample proteins and clinical parameters
None of the 23 proteins differentially expressed in EFA were associated with any of the clinical parameters investigated (maternal age, body mass index, estradiol levels, recovered oocytes, mature oocytes, numbers of fertilized, top-quality, and transferred embryos, day of ET, and number of gestational sacs) (data not shown). None of the five proteins studied by ELISA in blood samples was associated with any of the aforementioned parameters.
Overall safety
During the study period, 285 procedures were performed to aspirate endometrial fluid at the moment of ET. The pregnancy rate in these cases was similar to that in the control population (matched for age, infertility diagnosis, ovarian stimulation protocol, estradiol levels, and number of oocytes obtained, top-quality embryos, and embryos transferred) (Table 3).
No infectious or haemorrhagic complications occurred after ET in the EFA group or controls. Similarly, there were no infectious or haemorrhagic complications in the 200 oocyte donors who underwent endometrial fluid aspiration.
Discussion
In humans, embryo implantation occurs in the mid-secretory phase of the endometrial cycle, which is characterized by a number of changes in the endometrial epithelium and stroma, and especially by the development of endometrial glands. Endometrial secretions are essential for sustaining the conceptus prior to implantation [8, 9].
Despite the clear relevance to endometrial function, little is known about the identity of proteins secreted by the endometrium [4]. Since the early work of Noyes et al. [15], a number of histological and biochemical studies have focused on endometrial changes during the ovarian cycle. However, most endometrial histological or biochemical studies require an endometrial biopsy, precluding their performance in IVF cycles close to the moment when ET is to be carried out, as it could have a detrimental effect on implantation. In addition, the results of the biopsy from the stimulated cycle might not be the same as in a previous non-stimulated cycle, or even in a new stimulated cycle. Ovarian stimulation has been shown to be associated with an advancement of endometrial maturation regardless of the protocol used [16]. In contrast, endometrial fluid aspiration is an atraumatic procedure that, even if performed immediately prior to ET, does not affect implantation [17, 18]. We have previously shown how proteomic analysis of EFA detects more than 800 proteins [5, 6], and how the EFA proteomic pattern differs between patients with and without endometriosis [6].
A number of studies have been performed focusing on the so-called “receptive endometrium”. In most cases, the endometrium has been defined as receptive on the basis of histological, biochemical or genetic criteria. Nonetheless, the only way to ensure that an endometrium is receptive is that pregnancy has occurred in the very same cycle as when the analysed sample was taken. Thus, to avoid such bias, we have chosen to use the term “implantative” endometrium. Our embryos were not PGD tested, thus in our age range an aneuploidy rate close to 33% should be expected. Thus, our study could underdiagnose some cases where the endometrium could have been implantative if it had received an euploid embryo.
We observed that 23 proteins were significantly differentially expressed when comparing the group achieving versus non-achieving pregnancy. After excluding non-specific proteins, we were left with 20 proteins clearly differentially expressed in conception and non-conception cycles. Just one of these was up-regulated: catalase, with FC of 1.52–1.58. All the others were down-regulated (n = 19), with FC ranging from 0.31 to 0.67. The majority of differentially expressed proteins we found were related to the biological process of cell growth and/or maintenance (actin, F-actin capping subunit beta, cofilin, superoxide dismutase, stathmin) (Table 4). There were also a number of them related to energy pathways (arginase-1, catalase, glyceraldehyde-3- phosphate dehydrogenase) or protein metabolism (heat shock cognate 71 kDa and 70 kDa proteins, protein disulfide-isomerase, proteasome subunit beta, tubulin- specific chaperone A), and lastly, some were related to cell communication (annexin 2, cell division control protein 42 homolog and plastin 2).
Previously, some authors have applied large-scale proteomic techniques to study human endometrial receptivity by means of endometrial biopsies performed in different phases of the endometrial cycle [19,20,21,22]. Desouza et al. [20] comparing proliferative with secretory endometrial tissue, reported differential expression of a number of proteins. Some of these proteins (actin, cofilin, glyceraldehyde 3-phosphate dehydrogenase, heat shock cognate 71 kDa protein, and transferrin) we consistent with those we found differentially expressed in EFA [20]. Parmar et al. [21] also identified heat shock protein β-1 and transferrin to be up-regulated proteins in secretory endometrial tissue. Dominguez et al. [23.] focusing on “pre-receptive” and “receptive” endometrium found a number of proteins to be differentially expressed, but only annexin A2 and stathmin I were consistently up-regulated [23] It should be highlighted that in our study, both annexin 2 and stathmin 1 were significantly down-regulated. However, changes in intracellular protein concentration do not necessarily reflect simultaneous changes in protein secretion. Indeed, since these specific biomarkers of the receptive endometrium in the aforementioned studies were identified under natural cycles, it could be that they are not representative of stimulated IVF cycles [16].
Some of our results are consistent with those of a previous study in natural cycles [4] which analysed the endometrial lavage samples after flushing the uterine cavity, comparing pre-receptive and receptive endometrium [4]. Like them, we found lower expression of cofilin-1, glyceraldehyde-3-phosphate dehydrogenase and transferrin [4]. Some of the proteins we have found to be differentially expressed have also been described in the uterine fluid in the peri-implantation period in the cattle, namely, actin B, serotransferrin and HSP7C [24].
Annexin 2 is probably the most widely studied marker of implantation; it has been shown to increase in cultures of endometrial cells after interleukin 11 stimulation [25]. In a study investigating the endometrium of women using intrauterine devices for contraceptive purposes, annexin 2 was shown to be up-regulated in the receptive compared to pre-receptive endometrium [23]. Numerous studies have shown that annexin 2 is involved in cell adhesion and actin cytoskeletal rearrangements [26, 27], as well as increasing cell adhesion molecule production [28]. It has been suggested that annexin 2 could play a role in the remodelling of the apical pole of the luminal epithelium in the endometrium for cell-to-cell adhesion [23]. It should be highlighted that annexin 2 was down-regulated in conception cycles in our study. Concerning stathmin, this protein has been reported to be down-regulated in endometrial cells in receptive endometrium in transcriptomic- [29] and proteomic- [23] based studies, with a FC similar to that observed in our study.
When we tried to detect some of the EFA implantation markers in blood samples, none of the five proteins studied was significantly different in women achieving and not achieving pregnancy. In our opinion, this is a consequence of the limited effect of small changes in endometrial fluid on peripheral blood. We should recall that blood volume is about 1000 times greater than the endometrial volume (5.5 ml) [30], with the volume of endometrial fluid being considerably lower.
Finally, concerning safety, no impact was seen on PR and no infectious/haemorrhagic complications were detected among the 500 women where EFA was performed.
Our findings show that endometrial fluid is a protein-rich medium with a markedly different composition in conception and non-conception cycles, probably corresponding to a differential protein secretion that either facilitates embryo implantation and/or reflects a better endometrium quality. We conclude that a number of changes occur in protein composition of EFA in implantative cycles, most involving down-regulation, and measurement of these changes could constitute a useful tool for the ET planning.
References
Matorras R, Urquijo E, Mendoza R, Corcóstegui B, Expósito A, Rodríguez-Escudero FJ (2002) Ultrasound-guided embryo transfer improves pregnancy rates and increases the frequency of easy transfers. Hum Reprod 17:1762–1766
Friedler S, Schenker JG, Herman A, Lewin A (1996) The role of ultrasonography in the evaluation of endometrial receptivity following assisted reproductive treatments: a critical review. Hum Reprod Update 2:323–335
Valdez-Morales FJ, Gamboa-Domínguez A, Vital-Reyes VS, Cruz JC, Chimal-Monroy J, Franco-Murillo Y, Cerbón M (2015) Changes in receptivity epithelial cell markers of endometrium after ovarian stimulation treatments: its role during implantation window. Reprod Health 12:45–56
Scotchie JG, Fritz MA, Mocanu M, Lessey BA, Young SL (2009) Proteomic analysis of the luteal endometrial secretome. Reprod Sci 16:883–893
Casado-Vela J, Rodriguez-Suarez E, Iloro I, Ametzazurra A, Alkorta N, García-Velasco JA, Matorras R, Prieto B, González S, Nagore D, Simón L, Elortza F (2009) Comprehensive proteomic analysis of human endometrial fluid aspirate. J Proteome Res 8:4622–4632
Ametzazurra A, Matorras R, García-Velasco JA, Prieto B, Simón L, Martínez A, Nagore D (2009) Endometrial fluid is a specific and non-invasive biological sample for protein biomarker identification in endometriosis. Hum Reprod 24:954–965
Edgell TA, Rombauts LJ, Salamonsen LA (2013) Assessing receptivity in the endometrium: the need for a rapid, non-invasive test. Reprod Biomed Online 27:486–496
Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, Spencer TE (2001) Endometrial glands are required for preimplantation conceptus elongation and survival. Biol Reprod 64:1608–1613
Dunlap KA, Filant J, Hayashi K, Rucker EB 3rd, Song G, Deng JM, Behringer RR, DeMayo FJ, Lydon J, Jeong JW, Spencer TE (2011) Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod 85:386–396
Garrido-Gómez T, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simón C (2013) Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril 99:1078–1085
Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martínez-Conejero JA, Alamá P, Garrido N, Pellicer A, Simón C (2013) The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril 99:508–517
Vilella F, Ramirez LB, Simón C (2013) Lipidomics as an emerging tool to predict endometrial receptivity. Fertil Steril 99:1100–1106
Bourgain C, Devroey P (2003) The endometrium in stimulated cycles for IVF. Hum Reprod Update 9:515–522
Kolibianakis EM, Devroey P (2002) The luteal phase after ovarian stimulation. Reprod Biomed Online 5:26–35
Noyes RH, Hertig AT, Rock J (1950) Dating the endometrial biopsy. Fertil Steril 1:3–25
Haouzi D, Dechaud H, Assou S, De Vos J, Hamamah S (2012) Insights into human endometrial receptivity from transcriptomic and proteomic data. Reprod Biomed Online 24:23–34
van der Gaast MH, Beier-Hellwig K, Fauser BC, Beier HM, Macklon NS (2003) Endometrial secretion aspiration prior to embryo transfer does not reduce implantation rates. Reprod Biomed Online 7:105–109
Boomsma CM, Kavelaars A, Eijkemans MJ, Amarouchi K, Teklenburg G, Gutknecht D, Fauser BJ, Heijnen CJ, Macklon NS (2009) Cytokine profiling in endometrial secretions: a non-invasive window on endometrial receptivity. Reprod Biomed Online 18:85–94
Chen JI, Hannan NJ, Mak Y, Nicholls PK, Zhang J, Rainczuk A, Stanton PG, Robertson DM, Salamonsen LA, Stephens AN (2009) Proteomic characterization of midproliferative and midsecretory human endometrium. J Proteome Res 8:2032–2044
DeSouza L, Diehl G, Yang EC, Guo J, Rodrigues MJ, Romaschin AD, Colgan TJ, Siu KW (2005) Proteomic analysis of the proliferative and secretory phases of the human endometrium: protein identification and differential protein expression. Proteomics 5:270–328
Parmar T, Gadkar-Sable S, Savardekar L, Katkam R, Dharma S, Meherji P, Puri CP, Sachdeva G (2009) Protein profiling of human endometrial tissues in the midsecretory and proliferative phases of the menstrual cycle. Fertil Steril 92:1091–1103
Li J, Tan Z, Li MT, Liu YL, Liu Q, Gu XF, Zhou JZ, Zhuang GL (2006) Study of altered expression of annexin IV and human endometrial receptivity. Zhonghua Fu Chan KeZaZhi. 41:803–805
Domínguez F, Garrido-Gómez T, López JA, Camafeita E, Quiñonero A, Pellicer A, Simón C (2009) Proteomic analysis of the human receptive versus non-receptive endometrium using differential in-gel electrophoresis and MALDI-MS unveils stathmin 1 and annexin A2 as differentially regulated. Hum Reprod 24:2607–2617
Forde N, McGettigan PA, Mehta JP, O’Hara L, Mamo S, Bazer FW, Spencer TE, Lonergan P (2014) Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction 147:575–587
Yap J, Foo CF, Lee MY, Stanton PG, Dimitriadis E (2011) Proteomic analysis identifies interleukin 11 regulated plasma membrane proteins in human endometrial epithelial cells in vitro. Reprod Biol Endocrinol 9:73–87
Tressler RJ, Updyke TV, Yeatman T, Nicolson GL (1993) Extracellular annexin II is associated with divalent cation-dependent tumor cell-endothelial cell adhesion of metastatic RAW117 large-cell lymphoma cells. J Cell Biochem 53:265–276
Rescher U, Ludwig C, Konietzko V, Kharitonenkov A, Gerke V (2008) Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. J Cell Sci 121:2177–2185
Wolberg AS, Roubey RA (2005) Annexin A2: better left alone. Blood 105:1845–1846
Mirkin S, Arslan M, Churikov D, Corica A, Diaz JI, Williams S, Bocca S, Oehninger S (2005) In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod 20:2104–2117
Schild RL, Knobloch C, Dorn C, Fimmers R, van der Ven H, Hansmann M (2001) Endometrial receptivity in an in vitro fertilization program as assessed by spiral artery blood flow, endometrial thickness, endometrial volume, and uterine artery blood flow. Fertil Steril 75:361–366
Funding
This study was partially funded by a Grant for Fertility Innovation (GFI, 2011) from Merck, Darmstadt, Germany.
Author information
Authors and Affiliations
Contributions
RM: manuscript writing, supervision. SQ: data collection. BC: data collection. BP: supervision. AE: investigation, manuscript writing. RM: protocol. AR: supervision. DM: data collection. MF: protocol. FE: methodology. AA: methodology, validation, software. DN: methodology, validation, software
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have not conflict of interest.
Informed consent
We obtained approval from the Institutional Review Board (CEIC 09/54 and CEIC 11/45) and informed consent from participants.
Rights and permissions
About this article
Cite this article
Matorras, R., Quevedo, S., Corral, B. et al. Proteomic pattern of implantative human endometrial fluid in in vitro fertilization cycles. Arch Gynecol Obstet 297, 1577–1586 (2018). https://doi.org/10.1007/s00404-018-4753-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4753-1