Abstract
Objective
To explore the feasibility of three-dimensional (3D) transperineal ultrasound on the observation of paravaginal support in nulliparous and postpartum women.
Methods
Volume datasets were acquired in 50 nulliparous and 100 postpartum women using 3D transperineal ultrasound. Paravaginal supports were observed by studying the vaginal cross-sectional morphology. The extent of paravaginal support in specific level were evaluated by counting out at a 2 mm interval in tomographic ultrasound imaging mode in all subjects. The Mann–Whitney U test were applied to establish comparisons between the two groups.
Results
Three representative manifestations of vaginal cross-sectional morphology corresponding to different paravaginal support were presented from the dorsal side to the caudal side, both in nulliparous women and postpartum women. The extent of paravaginal support in middle vagina was 11 slices (range 9–12) in nulliparous women and 7 slices (range 4–10) in postpartum women (P < 0.05).
Conclusion
This pilot study confirmed that it was feasible to indirectly study paravaginal support by observing the vaginal cross-sectional morphology using 3D transperineal ultrasound.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pelvic floor dysfunction (PFD), mainly includes pelvic organ prolapse (POP) and stress urinary incontinence (SUI), is a common condition in parous women which increasingly affects women’s health and quality of life with the growth of age [1]. At present, the etiology of PFD still remains undetermined. Petros’s integral theory [2, 3] and DeLancey’s anatomic theory [4, 5] are relatively the two main theories of PFD. Both of the two theories confirmed that the support to the vaginal wall was a complex system involving the levator ani muscle, the arcus tendineus fascia pelvis (ATFP), the pubocervical fascia and the uterosacral/cardinal ligaments.
Imaging techniques, such as magnetic resonance imaging (MRI), three-dimensional (3D) transperineal ultrasound and tomographic ultrasound imaging (TUI) mode have been widely applied in the observation of PFD’s imaging feature, evaluation of therapeutic effect and exploration of the mechanisms associated with PFD [3, 6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Pelvic floor supporting structures, especially levator ani muscle, can be clearly shown by MRI in different imaging plane with its multiplanar imaging characteristics [14,15,16,17,18]. Meanwhile, with the advantages of low cost and handy operation, ultrasound also has extensive application in pelvic floor muscle imaging and achieved remarkable results [6,7,8,9,10,11,12,13], mainly in the field of levator ani muscle and levator hiatus. Many researches confirmed that women with POP were more likely to have levator ani muscle defect and larger levator hiatus size [7,8,9,10]. With the advantage of multi-planar display of TUI mode, the demonstration of levator ani muscle in multi-continuous-plane in one screen made it more easily to detect avulsion [12, 13].
However, pelvic floor connective tissue, another important part of the pelvic floor support system, seemed to be much harder to be imaged. Although Tunn et al. found that endopelvic fascia could be clearly visible at middle and proximal urethra level in some nulliparous women by MRI, few reports involving ultrasound imaging of pelvic floor connective tissue were available so far [19]. Anatomically, pelvic floor connective tissue is different from other pelvic structures with fixed morphology, such as pelvic organs or pelvic floor muscle, diffusedly distributing with unclear boundary. In DeLancey’s anatomic theory, the connective tissue surrounded vagina, which was part of pelvic floor connective tissue, known as ‘paravaginal support’, was described in detail and divided into three different levels [4, 5]. It was suggested further that different defect of ‘paravaginal support’ at different level might cause different type of PFD correspondingly [2,3,4,5]. Considering the relationship of vaginal morphology and its surrounding connective tissue suggested by DeLancey in the anatomic theory, we attempted to find out whether the ‘paravaginal support’ proposed by DeLancey could be indirectly displayed by observing the vaginal cross-sectional morphology with 3D transperineal ultrasound. In addition, we tried to find out the feasibility of TUI mode of 3D transperineal ultrasound in observing the extent variety of paravaginal support by comparing the difference of nulliparous women and postpartum women without severe PFD.
Patients and methods
This study was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and written informed consent was provided by all participants. A total of 50 nulliparous women in gynecological clinic with irregular menstruation, vaginitis, pelvic inflammatory disease or infertility were recruited. A total of 100 postpartum women who gave birth between December 2016 and February 2017 in the Department of obstetrics were also recruited. Inclusion criterions for postpartum women were an uncomplicated singleton pregnancy, at 37–42 weeks’ gestation and first delivery. The exclusion criterion for both nulliparous women and postpartum women were pelvic tumor with the diameter ≥ 3.0 cm (such as uterine myoma, ovarian tumor and fallopian tube tumor) and women with a history of pelvic trauma or surgery, chronic cough or constipation leading to increase in abdominal pressure and BMI ≥ 28. All the participants were required to fill in the questionnaire about the physical condition, the symptoms of pelvic floor and urinary incontinence. Physical examination for prolapse (International Continence Society POP-Q system) was also performed. POP-Q grade 2 or higher refers to leading edge of prolapse situated below 1 cm above hymen [23]. Severity of stress urinary incontinence (SUI) was graded as Grade I, Grade II and Grade III according to the Ingelman-Sundberg scale [24] and the specific methods were as follows: Grade I, urinary incontinence while coughing or sneezing; Grade II, urinary incontinence while running or picking up objects from the floor; and Grade III, incontinence while walking or climbing stairs. Women with significantly SUI (Grade II or higher) and significantly objective prolapse (International Continence Society POP quantification, grade 2 or higher) were excluded.
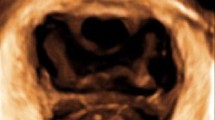
Subsequently, all the participants underwent 3D translabial ultrasound examination using GE Voluson E8 (GE Medical system, Zipf, Australia) with RAB4-8-D 3D volume probe. Ultrasound volumes of postpartum women were acquired at 30–90 days (in 3 months) after delivery. Imaging was performed on women after emptying their bladder on supine position at rest. Ultrasound data was analyzed offline using GE 4D View software by an experienced doctor, who was blinded against all clinical information. The cross-sectional morphology of vagina was reconstructed by the volume data obtained in mid-sagittal plane perpendicular to the long axis of vagina (Fig. 1). The whole vagina from cranial to caudal was browsed. Then, continuous slices at a 2 mm interval of the cross-sectional plane perpendicular to the long axis of vagina were assessed and the extent of paravaginal support were evaluated by TUI mode (Fig. 2). The ultrasonic manifestations of vaginal cross-sectional morphology in nulliparous women and postpartum women were observed and the differences were compared. Paravaginal support in all subjects were observed indirectly by studying the vaginal cross-sectional morphology.
Statistical analysis was performed using SPSS 13.0 for Windows (SPSS Chicago, IL, USA). Median values were compared by Mann–Whitney U test and Chi square test, and value of P < 0.05 was considered statistically significant.
Results
The average age was 25 years (range 20–29 years) in nulliparous women and 27 years (range 21–35 years) in postpartum women. Basic clinical information of 100 postpartum women is shown in Table 1. Twenty-two of the 100 postpartum women (22%) suffered from Grade I SUI during pregnancy or after delivery. None of them were with persistent SUI from antepartum to postpartum.
The manifestations of vaginal cross-sectional morphology in nulliparous and postpartum women
The plane perpendicular to the axis of vagina was obtained after post-processing of the 3D volume data (Fig. 1), in which three representative manifestations of vaginal cross-sectional morphology at different vaginal levels were presented from the dorsal side to the caudal side in nulliparous women (Fig. 2a–c). In postpartum women, the images of different vaginal cross-sectional morphology were also obtained (Fig. 2d–f), a little bit more blurred than those of nulliparous women.
In the proximal vagina, vagina was flat-bar-like, with rounded ends (Fig. 2a, d). In the middle vagina, vagina was box-like shaped and the urethra was located directly ahead of the anterior vaginal wall, with a clear division between them (Fig. 2b, e). In the distal vagina, vagina appeared as crescent-like, forward wrapped around the urethra, with blurring boundaries between vaginal anterior wall and posterior urethral wall (Fig. 2c, f).
Among manifestations of the entire vagina, only the middle vagina was shown in full-length, while both cross-sectional images of proximal and distal vagina were relatively clearer only in the part adjacent to the middle vagina. The parts of the most distal vagina (close to the probe part) and the proximal end (near cervical part) were shown unclearly.
The extent of paravaginal support in the middle vagina in TUI mode
The vaginal cross-sectional planes between the vaginal external cervical orifice and the external orifice were displayed continuously at a 2 mm interval in TUI mode both in nulliparous women and postpartum women (Fig. 3). The cross-sectional images of the middle vagina, which described as box-like shaped above, were totally shown in full-length and clearly, while display of the most proximal and most distal vagina was poor and it was relatively clearer only in the part adjacent to the middle vagina.
To evaluate the extent of paravaginal support in the middle vagina, the slices of images with box-like shaped vaginal cross-sectional morphology were counted out both in nulliparous women and postpartum women. In nulliparous group, the median of the extent of paravaginal support in the middle vagina was 11 slices (range 9–12), while it was 7 slices (range 4–10) in postpartum group, significantly reduced. The difference was of statistical significance (P < 0.05). The median of the extent of paravaginal support in the middle vagina for CS group and vaginal delivery group both were 7 slices (range 4–10), with no statistical significant difference (P > 0.05) (Table 2).
Discussion
Paravaginal defect, injury of pelvic connective tissue, which represents a detachment of the anterior vaginal wall and the arcus tendineus fascia pelvis (ATFP) anatomically, can only be seen directly by observing it through the space of Retzius [25,26,27]. In 1990s, researchers firs attempted to indirectly learn paravaginal support defects by observing the shape of the full bladder with transabdominal ultrasound. When one side of the bladder projecting outward on the transverse image, it was considered as vaginal wall support defects on this side [28,29,30]. Some MRI researches found that women with PFD might lose their typical configuration of the vagina without levator ani muscle defect, and speculated that the disruption of connective tissue from the pelvic sidewall might be the cause [31, 32]. However, few researches have studied the images of the cross-sectional morphology of entire vagina and its paravaginal support so far. In this study, we successfully applied 3D transperineal ultrasound, a technique which is good at displaying the cross-sectional images of the pelvic floor, in imaging the vaginal cross-sectional morphology, and generalized three representative manifestations of vaginal cross-sectional morphology at different vaginal levels from the dorsal side to the caudal side, both in nulliparous women and postpartum women.
Vagina is a fibromuscular pipe structure, pulled by the connective tissue around its wall to shape to fixed morphology. Once the traction direction of the vaginal wall was different, the vaginal morphology was then different correspondingly. In Delancey’s anatomic theory, paravaginal support was suggested to support the vagina at three different levels owing to the different lateral connective tissue at different levels [4, 5]. Level I, the cephalic 2–3 cm of the vagina, equivalent to the proximal vagina, was supported by the uterosacral and cardinal ligaments. The vagina was then pulled back and upwards through their attachments to the cervix and upper vagina. In our study, it was shown as a flat-bar-like shape with rounded ends. Level II, the middle vagina, was supported by pubocervical fascia. The vagina wall was pulled by the surrounding connective tissue in four directions, including left anterior, left posterior, right anterior and right posterior, attached to the ATFP. In our study, it was shown as a box-like shape. Some researchers had previously described the vagina as a cross-sectional H shape in the upper urethra plane by magnetic resonance imaging [21, 31, 32], which were similar to our results. Level III, the caudal 2–3 cm above the hymeneal ring, equivalent to the distal vagina, was supported by perineal membrane. The vagina was fused with the surrounding structures including urethra, perineal membrane, and levator ani. In our study, it was crescent-like shaped, with blurring boundaries between vaginal anterior wall and posterior urethral wall. As a result, it seemed feasible to learn the paravaginal support at different levels indirectly by observing the vaginal cross-sectional morphology correspondingly. The different vaginal cross-sectional morphology at different levels indicated the existence of corresponding paravaginal support at different levels.
Specifically speaking, level II was the easiest to be entirely displayed. The position of level I was too distant from the perineum to detect for the 3D transperineal ultrasound, which made it hard to clearly display this whole level. As for level III, it was relatively too close to the probe and to avoid the pressure from the probe on level III, which made it much difficult to display clearly. However, level II not only ensured the appropriate detect depth requirement for scanning but also escaped the inevitable pressure from the probe, which allowed 3D transperineal ultrasound to perfectly display the level. So did TUI mode. As a result, we further studied the extent of level II instead of level I and level III.
At present, TUI mode was mainly applied in the observation of the lesion extent, such as levator trauma [13]. In this study, we found TUI mode an effective method to roughly learn the extent of level II by evaluating the full length of the middle vagina. It showed that the extent of level II was 11 slices (range 9–12) at a 2 mm interval in nulliparous women and 7 slices (range 4–10) in postpartum women. The extent variety of level II in nulliparous and postpartum women without severe PFD was also shown distinctly. However, the cause of the descent extent and wider range of level II in postpartum women, and its correlation to pelvic floor function still need to be further studied. In addition, our results showed that there was no difference of the median and range of level II extent between women experienced cesarean section and vaginal delivery. Since this postpartum group in our study only included the postpartum population without PFD, we suggested that the result should not be simply interpreted as vaginal delivery with no effect on level II. Further improvement in the selection of the study subjects would be required to confirm the effect of childbirth on level II.
In conclusion, 3D transperineal ultrasound is a valid and convenient way to indirectly observe the paravaginal support. Paravaginal support at different levels can be learned from different vaginal cross-sectional morphology correspondingly. TUI mode can be used to learn the extent of level II and its variety. We successfully evaluated the existence of paravaginal support by 3D transperineal ultrasound, and generally learned the normal range of level II in this study, expecting that this research would be helpful for the study of level II function in the future.
References
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk DE, Sand PK, Schaer GN (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. International Urogynecological Association; International Continence Society. Neurourol Urodyn 29(1):4–20
Papa Petros P, Ulmsten U (1990) An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand 153:7–31
Petros PEP, Woodman PJ (2008) The integral theory of continence. Int Urogynecol J Pelvic Floor Dysfunct 19:35–40
DeLancey JO (1992) Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166:1717–1724
DeLancey J (1994) Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol 170:1713–1720
van Delft K, Sultan AH, Thakar R, Schwertner-Tiepelmann N, Kluivers K (2014) The relationship between postpartum levator ani muscle avulsion and signs and symptoms of pelvic floor dysfunction. BJOG 121(9):1164–1171
Dietz HP, Pattillo Garnham A, Rojas Guzmán (2017) Is it necessary to diagnose levator avulsion on pelvic floor muscle contraction? Ultrasound Obstet Gynecol 49(2):252–256
Ying T, Li Q, Xu L, Liu F, Hu B (2012) Three-dimensional ultrasound appearance of pelvic floor in nulliparous women and pelvic organ prolapse women. Int J Med Sci 9(10):894–900
Dietz HP, Shek C, De Leon J, Steensma AB (2008) Ballooning of the levator hiatus. Ultrasound Obstet Gynecol 31(6):676–680
Dietz HP, Franco AV, Shek KL, Kirby A (2012) Avulsion injury and levator hiatal ballooning: two independent risk factors for prolapse? An observational study. Acta Obstet Gynecol Scand 91(2):211–214
Rostaminia G, White D, Hegde A, Quiroz LH, Davila GW, Shobeiri SA (2013) Levator ani deficiency and pelvic organ prolapse severity. Obstet Gynecol 121(5):1017–1024
Dietz HP, Severino M, Kamisan Atan I, Shek KL, Rojas Guzman (2016) Warping of the levator hiatus: how significant is it? Ultrasound Obstet Gynecol 48(2):239–242
Dietz HP, Shek KL (2009) Tomographic ultrasound imaging of the pelvic floor: which levels matter most? Ultrasound Obstet Gynecol 33(6):698–703
Yan Y, Dou C, Wang X, Xi Y, Hu B, Ma L, Ying T (2017) Combination of tomographic ultrasound imaging and three-dimensional magnetic resonance imaging-based model to diagnose postpartum levator avulsion. Sci Rep 7(1):11235
Dumoulin C, Peng Q, Stodkilde-Jorgensen H, Shishido K, Constantinou C (2007) Changes in levator ani anatomical configuration following physiotherapy in women with stress urinary incontinence. J Urol 178(3 Pt 1):970–977
Khatri G, Carmel ME, Bailey AA, Foreman MR, Brewington CC, Zimmern PE, Pedrosa I (2016) Postoperative imaging after surgical repair for pelvic floor dysfunction. Radiographics 36(4):1233–1256
Betschart C, Kim J, Miller JM, Ashton-Miller JA, DeLancey JO (2014) Comparison of muscle fiber directions between different levator ani muscle subdivisions: in vivo MRI measurements in women. Int Urogynecol J 25(9):1263–1268
Schofield MLA, Higgs P, Hawnaur JM (2005) MRI findings following laparoscopic sacrocolpopexy. Clin Radiol 60(3):333–339
Tunn R, Delancey JO, Howard D, Ashton-Miller JA, Quint LE (2003) Anatomic variations in the levator ani muscle, endopelvic fascia, and urethra in nulliparas evaluated by magnetic resonance imaging. Am J Obstet Gynecol 188(1):116–121
van Delft K, Thakar R, Sultan AH, Kluivers KB (2015) Does the prevalence of levator ani muscle avulsion differ when assessed using tomographic ultrasound imaging at rest vs on maximum pelvic floor muscle contraction? Ultrasound Obstet Gynecol 46(1):99–103
Macura KJ (2006) Magnetic resonance imaging of pelvic floor defects in women. Top Magn Reson Imaging 17(6):417–426
Athanasiou S, Chaliha C, Toozs-Hobson P, Salvatore S, Khullar V, Cardozo L (2007) Direct imaging of the pelvic floor muscles using two-dimensional ultrasound: a comparison of women with urogenital prolapse versus controls. BJOG 114(7):882–888
Haylen BT, Maher CF, Barber MD, Camargo S, Dandolu V, Digesu A, Goldman HB, Huser M, Milani AL, Moran PA, Schaer GN, Withagen MI (2016) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecol J Pelvic Floor Dysfunct 27(4):655–684
Ingelman-Sundberg A, Ulmsten U (1983) Surgical treatment of female urinary stress incontinence. Contrib Gynecol Obstet 10:51–69
Richardson AC, Edmonds PB, Williams NL (1981) Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol 57(3):357–362
Richardson AC, Lyon JB, Williams NL (1976) A new look at pelvic relaxation. Am J Obstet Gynecol 126(5):568–573
White GR (1909) Cystocele—a radical cure by suturing lateral sulci of the vagina to the white line of pelvic fascia. Int Urogynecol J Pelvic Floor Dysfunct 8(5):288–292
Martan A, Masata J, Halaska M, Otcenásek M, Svabik K (2002) Ultrasound imaging of paravaginal defects in women with stress incontinence before and after paravaginal defect repair. Ultrasound Obstet Gynecol 19(5):496–500
Nguyen JK, Hall CD, Taber E, Bhatia NN (2000) Sonographic diagnosis of paravaginal defects: a standardization of technique. Int Urogynecol J Pelvic Floor Dysfunct 11(6):341–345
Ostrzenski A, Osborne NG (1998) Ultrasonography as a screening tool for paravaginal defects in women with stress incontinence: a pilot study. Int Urogynecol J Pelvic Floor Dysfunct 9(4):195–199
Huebner M, Margulies RU, DeLancey JO (2008) Pelvic architectural distortion is associated with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 19(6):863–867
Tillack AA, Joe BN, Yeh BM, Jun SL, Kornak J, Zhao S, Deng D (2015) Vaginal shape at resting pelvic MRI: predictor of pelvic floor weakness? Clin Imaging 39(2):285–288
Acknowledgements
National Science Foundation of China is acknowledged for providing financial support to this work (No. 81571699).
Author information
Authors and Affiliations
Contributions
CD: project development, manuscript writing, data collection. QL: project development, data collection, manuscript writing. TY: conceptualization, funding acquisition. WS: data collection. YY: methodology. YL: data analysis. XW: data analysis.
Corresponding authors
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. All procedures performed in the study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments.
Conflict of interest
We declare that there are no conflict of interest.
Rights and permissions
About this article
Cite this article
Dou, C., Li, Q., Ying, T. et al. Value of transperineal ultrasound on the observation of paravaginal support. Arch Gynecol Obstet 297, 943–949 (2018). https://doi.org/10.1007/s00404-018-4659-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4659-y