Abstract
Objective
Ovarian cancer is the fifth most common cancer in women and one of the leading causes of death from gynecological malignancies. Despite of its clinical importance, ovarian tumorigenesis is poorly understood and prognosis remains poor. This is particularly true for the most common type of ovarian cancer, high-grade serous ovarian cancer.
Results
Two models are considered, whether it arises from the ovarian surface epithelium or from the fallopian tube. The first model is based on (1) the pro-inflammatory environment caused by ovulation events, (2) the expression pattern of ovarian inclusion cysts, and (3) biomarkers that are shared by the ovarian surface epithelium and malignant growth. The model suggesting a non-ovarian origin is based on (1) tubal precursor lesions, (2) genetic evidence of BRCA1/2 mutation carriers, and (3) recent animal studies. Neither model has clearly demonstrated superiority over the other. Therefore, one can speculate that high-grade serous ovarian cancer may arise from two different sites that undergo similar changes. Both tissues are derived from the same embryologic origin, which may explain how progenitor cells from different sites can respond similar to stimuli within the ovaries. However, distinct molecular drivers, such as BRCA deficiency, may still preferentially arise from one site of origin as precancerous mutations are frequently seen in the fallopian tube.
Conclusions
Confirming the origin of ovarian cancer has important clinical implications when deciding on cancer risk-reducing prophylactic surgery. It will be important to identify key biomarker to uncover the sequence of ovarian tumorigenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer (OC) is the leading cause of death from gynaecological malignancies. The disease consists of multiple subtypes, of which epithelial OCs account for about 90% of cases [1, 2]. These epithelial OC subtypes include endometrioid, clear cell, mucinous, and serous OC, all of which have been suggested to depend on unique sequences of tumorigenesis [3,4,5,6]. In Germany, approximately 8000 women are diagnosed with OC and about 5500 women die every year from the disease [7]; of which high-grade serous OC (HGSOC) represent the most common type with about 70% of all cases. Most patients (about 80%) that present with advanced OC are diagnosed with HGSOC [8]. HGSOC differs from low-grade serous OC (LGSOC) by having a higher mitotic index, a more aggressive behaviour and typically correlates with a poorer prognosis [9]. LGSOC is thought to sequentially arise from serous cystadenoma and borderline serous OC to invasive micropapillary serous borderline tumors, representing a distinct disease entity from HGSOC, associated with unique characteristic mutations [6].
Despite the clinical burden of the disease, the site of origin of HGSOC is still debated [10, 11]. This is in stark contrast to the well-established sequence of tumorigenesis in other tumors, such as colorectal cancer [12]. Here, the discovery that the detection of precancerous colonic polyps correlates with clearly defined neoplastic changes has transformed diagnosis and management of colorectal cancer [13]. One could speculate that the dearth of new innovative clinical therapies may be at least partially due to the unresolved sequence of HGSOC growth [7, 14].
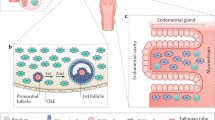
What are the main sites of origin of HGSOC that are debated? On the one hand, evidence suggests that this cancer arises from the ovarian surface epithelium (OSE), which is related to the mesothelium of the peritoneum [15]. It is loosely attached to the underlying ovarian stroma and separated by the tunica albuginea. The OSE harbours stem cells, which may contribute to tumor formation (Fig. 1). On the other hand, it has been suggested that HGSOC originates from the fallopian tubes, which consist of differentiated columnar epithelium composed of ciliary and secretory cells (Fig. 2). It has been suggested that the fimbriae of the fallopian tube deposit cancer cells onto the ipsilateral ovary promoting tumor formation [16].
Development of HGSOC arising from the OSE. The diagram shows the stepwise progression to HGSOC from the formation of ovarian inclusion cysts, their change in gene expression, and the malignant transformation, which can ultimately lead to cancer formation. Genotoxic stress through repetitive ovulatory inflammation responses may lead to malignant transformation, which is likely to be promoted by deregulation of the pluripotent stem cell activity within the OSE and/or ovarian inclusion cysts
Progression of normal fallopian tube epithelium to invasive HGSOC. The fallopian tube epithelium is composed of a single layer of ciliated and secretory cells that are exposed to ovulation-associated inflammatory cytokines. This repetitive genotoxic stress causes DNA damage and induces p53 mutation, leading to the clonal expansion of physiologically normal appearing epithelial cells, termed p53 signature. Further mutations enable cells to acquire a proliferative capacity, giving rise to serous tubal intraepithelial carcinoma (STIC). As STICs progress, invasive cancer cells are exfoliated from the fimbriae, whereupon they may spread rapidly to the surface of the ovary and may establish tumour formation and transformation to HGSOC. Exfoliation may also occur from STICs prior to invasion of the fimbrae (the relative size of the fallopian tube and the ovary is not representative)
There are several reasons why the origin of HGSOC remained obscure. Unlike almost all other epithelial-derived tumors, HGSOC in situ is rarely found in ovaries and the identification of precursor lesions is remarkably infrequent, hindering the ability to accurately map the origin of the disease in the host tissue. The controversy of the origin of HGSOC has made it challenging to implement effective screening, prevention or develop novel therapeutic strategies for this disease. This article reviews the fascinating new insights in ovarian tumorigenesis, models of ovarian tumorigenesis, and discusses whether HGSOC originates from the OSE or the fallopian tube.
Ovarian surface epithelium model
The OSE model was first proposed by Fathalla [17]. The total number of ovulation events is thought to contribute to tumorigenesis by promoting a pro-inflammatory microenvironment and activation of DNA double-strand breaks (DDSBs) and subsequent repair or inadequate repair in the case of cancer growth. This is based on: firstly, the reduced risk of HGSOC formation that is associated with the long-term intake of the contraceptive (estrogen-containing) pill due to regulating ovulation [18]. Secondly, HGSOC risk is strongly associated with BRCA1/2 mutations [19, 20]. Approximately 50% of HGSOCs are characterized by dysfunction of the homologous recombination (HR) pathways, mainly because of BRCA1/2 mutations or loss of other factors of HR [21]. The identification of the BRCA1 gene has been key in the understanding of the genetic susceptibility of ovarian (and breast) cancer [22].
Mutations in BRCA1/2 impair the efficacy of DDSB repair and thus promote cancer growth, including OC [23, 24]. An international observational study of 31,481 patients with confirmed BRCA1/2 carrier status analyzed the risk of OC. This has shown that women with mutations in BRCA1/2 have a statistically significantly increased risk of OC, with an overall absolute risk of 34% for BRCA1 mutation carriers and 11% for BRCA2 mutation carriers [19, 25]. In contrast, the risk of developing OC in the general (German) population is about 0.7% by the age of 75 [7].
It can be argued that the repeated self-healing process after ovulation may increase the frequency of inadequate DDSB repair event mistakes due to malfunctioning in the repair machinery in BRCA1/2-mutation carriers, which in turn increases genotoxic stress and promotes malignant transformation of the OSE (Fig. 1).
The OSE harbor stem cells, which may suggest that dysregulated pluripotency of these stem cells, may facilitate tumor growth, particularly, under cyclical inflammatory response. It was described that stem cell maintenance activity is silenced in cancerous OSE, suggesting that deregulation contributes to HGSOC (Fig. 1) [26]. There is strong evidence from other cancers, such as colorectal cancer, suggesting that inadequate host tissue stem cell activity gives rise to malignant transformation and growth [27, 28]. A process might potentially also contribute to ovarian tumorigenesis.
It has been considered that the OSE-lined inclusion cysts are early metaplastic transformations, which would support the OSE model, because these cysts exhibit a high degree of oncogenic potential in otherwise pathologically normal ovaries (Fig. 1) [29]. However, there is also a high frequency of inclusion cysts in pathologically normal ovaries [30], and it cannot be ruled out that metastatic cancer cells, deposit onto ovaries, may promote the formation of these cysts. Despite the ovaries being the site of the disease at later stages and often at the time of clinical presentation, it has been argued that the OSE is not the actual tissue of origin of OC.
Fallopian tube model
Although it seems controversial to suggest extra-ovarian cells as the origin for HGSOC, the physiology of the female genital tract and its common types of malignancies may explain the reasoning behind this approach. The fallopian tube model was first proposed by Dubaeu [31]. A strong link between the fallopian tube and HGSOC has been suggested, because irrespective of family history, about 67% of ovarian carcinomas have also been shown to have coexisting tubal lesions [32]. Kurman and Shih further suggested that HGSOC, compared to low-grade serous, endometrioid, mucinous, or clear-cell OCs, typically arises from precursor lesions in the fallopian tube, called serous tubal intraepithelial carcinoma (STIC) [33, 34].
A histopathological study suggested a direction of this spread (Fig. 2) [35]. The histology of most HGSOC samples bears little resemblance to the OSE and ovarian tissue, but recapitulates the histological features of Müllerian epithelium that is present in the fallopian tube. In 2001, Piek et al. provided histopathological evidence that HGSOC may originate from the fallopian tube (Fig. 2) [35, 36]. They reported the presence of dysplastic changes in the fallopian tube in 11 of 12 prophylactic salpingo-oophorectomy specimens removed from suspected BRCA-mutation carriers, whereas none was identified in control subjects, i.e., not carrying BRCA mutations [35, 36]. It was shown that HGSOC is characterized by harbouring mutations in the tumor suppressor gene TP53 in the vast majority of cases [21, 37]. This is known as the p53 signature. Recent sequencing of HGSOCs has further confirmed that more than 95% of tumors have mutations in TP53, which is significantly different from other epithelial OCs (p value < 0.0005) [38, 39].
In contrast to BRCA mutation carriers, it remains unknown whether the occurrence of the p53 signature correlates with an increased risk of HGSOC in women without BRCA1/2 mutations and whether a highly selective patient cohort overestimates the role of the p53 signature.
To explore the role of the p53 signature, Lee et al. identified that the changes identified in the tumor suppressor gene p53 in tubal epithelial cells resembled the mutations seen in STICs with morphologically intermediates between both stages [40]. Recent studies of ovarian tumorigenesis in an animal model also provided strong evidence for the fallopian tube model [16]. Importantly, early removal of the fallopian tube prevented ipsilateral OC formation in mice [16]. A meta-analysis has also shown that BRCA1/2 mutation carriers who had undergone risk-reducing salpingo-oophorectomy have a more than 50% risk reduction of developing ovarian or fallopian tube cancer [41].
This would suggest that the fallopian tube may likely be the origin of at least a substantial number of HGSOC, particularly, in BRCA1/2 mutation carriers. However, to more accurately understand the sequence and origin of ovarian tumorigenesis, it is essential to identify key biomarkers and molecular drivers that promote HGSOC growth [42, 43]. A recently suggested biomarker could be the epithelial stem cell marker leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) [44]. This marker has recently been shown to be expressed in stem progenitor cells of the OSE and tubal epithelia [45].
LGR5/6-positive cells as the cellular marker of the origin of ovarian cancer
Lgr5 is member of the Wnt signalling pathway, acting as receptor for a Wnt pathway agonist R-Spondin [44, 46]. Lgr5 was first discovered in stem cells of the intestine and colon in 2007 and later described a common marker in other epithelial stem cells [27, 44, 47], linking it to cancer growth in various cancers of epithelial origin [27, 48]. High Lgr5 expression has also been demonstrated to correlate with poor prognosis and advanced tumor stage in patients with OC [49]. Both sites of HGSOC are epithelial in origin, making Lgr5-positive (stem) cells an obvious candidate to contribute to ovarian tumorigenesis. Lgr5 expression has been shown in the OSE and tubal epithelium [45]. Lgr5 has been found in ovarian stem cell populations suggesting a role in ovarian stem cell regeneration and may shed light onto tumorigenesis of OSE- or tubal-derived OCs [50].
Interestingly, a recent study has shown that tubal organoids can grow in culture over several months, relying on growth factors similar to intestinal organoids. This would allow to study the stepwise progression of STICs and to identify the molecular and pathological changes that result in the formation of HGSOC. This could be achieved by selectively inducing mutations that are believed to be involved in the early stages of the disease. If Lgr5-positive cells drive regeneration of the OSE and contribute to the stemness of the fallopian tube, this may explain how the two different sites contribute to ovarian tumorigenesis by relying on similar pathways for cell growth. De-differentiated progenitor cells from the fallopian tube could receive a further growth stimulation once spread onto the OSE. On the other hand, Lgr5 expression has been shown to be present in the OSE, specifically at the cleft region of growing follicle and rupturing OSE [45]. Contributing to the cyclical self-renewal process of the OSE would predestine OC growth to be at least partially dependent on Lgr5/6-positive stem cells. More recently, a study demonstrated that human three-dimensional fallopian tube cultures could grow in laboratory conditions for many months, requiring similar signalling/growth factors to intestinal organoid cultures [51]. Intriguingly, this study suggested that Lgr5 expression was not significantly increased in fallopian tubal epithelium, whereas Lgr6 expression has been suggested as the key marker for tubal self-renewal. However, the underlying (Wnt) pathway remains the same for Lgr5- or Lgr6-positive cells, suggesting a role of Lgr5/6-positive stem cells [45, 51]. In turn, this would suggest that the fallopian tubal epithelium and the OSE share a common self-renewal mechanism.
It will be important to identify the molecular steps that lead to a loss of appropriate stem cell function and promote distinct changes in gene expression in the OSE and tubal epithelium. This could also shed light on the well-established link between (sporadic and hereditary) gynaecological and colorectal cancer, which is typically seen in hereditary non-polyposis colon cancer (Lynch syndrome) [52].
Discussion
It is remarkable that despite decades of research the cells of origin of HGSOC have not been identified. This could be explained, because the two models are supported by unique yet equally valid findings, and there is no evidence that clearly excludes one of the two models. However, in BRCA mutation carriers, the presence of a p53 signature in the fallopian tubes may strongly suggest that, at least in this patient cohort, a tubal origin of HGSOC seems favourable [35, 36], reflecting proven cancer risk reduction and the advice of prophylactic risk-reducing salpingo-oophorectomy in these individuals [53].
If both models are true, the question remains of how the ovarian tissue environment forms a hotspot for cancer formation from (at least) two different origins [14]. This could (at least partially) be explained developmentally, because the OSE and the fallopian tube share the same embryonic origin. Therefore, one could imagine that ovaries offer the same growth-promoting environment for cells form both tissue types, which would explain the similarities of HGSOCs that arise from different origins.
Interestingly, risk factors for tubal and ovarian origin of HGSOC have been shown to differ by looking at the tumor dominance as a surrogate for the cell of origin. This study, based on a case–control study (New England Case–Control Study) and two cohort studies (Nurses’ Health Study/Nurses’ Health Study II), classified dominant tumors, as being either restricted to one ovary or at least twice as large on one ovary than on the other [54]. These dominant tumors were thought to arise from the ovary, whereas non-dominant tumors were thought to arise from the fallopian tube. Two or more pregnancies were more likely to be associated with a tubal origin, whereas endometriosis and age were more likely to be associated with an ovarian origin of cancer [54].
Neither of the two models can uniquely explain how cancer cells grow into or reach the ovarian tissue and how these cells, once established in the ovary, are stimulated to rapidly form HGSOC. One could speculate that the rapid tumor growth relies on the growth-stimulating environment established by the OSE. Likewise, it has also been suggested that endocrine dysregulation and/or inadequate hormonal exposure is linked to ovarian tumorigenesis [55]. This hypothesis stems from the increased risk of ovarian cancer after hormonal replacement therapy and the potential role of sex hormone receptors in ovarian tumorigenesis [55, 56].
It is also known that tumor heterogeneity is widespread, having been already described in OC more than 30 years ago [57, 58]. One could assume that ovarian tumor heterogeneity may also partially reflect the tissue origin of HGSOC. Recent advances in culturing normal human epithelial cells from the fallopian tube and ovaries from the same donors argue that ovarian tumor heterogeneity may reflect the different cells of origin [59]. However, this study relied on immortalized cell lines with ectopic hTERT expression [59]. Interestingly, a recent publication compared frequently used OC cell lines and compared the genomic profile of cell lines to 500 tissue samples from HGSOC [60]. This alarmingly suggested that particularly, the most frequently used OC cell lines (SKOV3 and A2780) bear little molecular resemblance to the actual disease, i.e., most significantly not harbouring TP53 mutations [60]. Importantly, a newly published technique showed promising results, describing the routine isolation of primary cell lines from human OC with > 95% efficiency [61]. The authors described the isolation of 25 new OC cell lines that showed constant growth in a newly developed cell culture medium while maintaining their genomic profile. It remains to be seen whether this new culturing technique may allow the establishment of more representative and closely disease-related cell cultures, avoiding the alterations inadvertently seen in long-term cultured cancer cell lines.
Although a significant number of women present with bilateral HGSOC, it is still poorly understood how cancer arises at both ovaries at the same time or whether the same precursor lesion spreads to the contralateral ovary. Interestingly, the mouse model described by Kim et al. shows that there is no cancer formation after removal of the ipsilateral fallopian tube [16]. This model may be utilized to identify signaling pathways that may contribute to bilateral OC formation.
The understanding of LGR5/6-positive stem cells in regulating the stemness of the OSE and fallopian tubal epithelium may shed light onto the origin of HGSOC. If both epithelia rely on the same key mechanism for self-renewal, this could explain how precancerous tubal lesions may give rise to HGSOC once implanted on the OSE. Using tubal (and ovarian) organoids may aid to model the origin of the disease.
Conclusion
It is evident that some OCs are a result of p53 inactivation and mutations in the epithelial cell lining of the fallopian tube, from which cancerous cells are then subsequently deposit onto the ovaries and promote cancer formation. This is particularly true for the subset of HGSOCs that arise in BRCA mutation carriers. However, it is unclear whether the OSE may contribute to this process or whether the OSE itself undergoes metaplastic changes and gives rise to HGSOC independently. Both models (OSE and fallopian tube) offer compelling evidence for the origin of HGSOC and future studies on the biology of STICs, and the OSE and biomarkers may shed light onto how HGSOC originates and how the tissue microenvironment itself drives ovarian tumorigenesis. Ovarian and tubal organoids represent a promising tool to investigate the influence of the tubal epithelium or the OSE contributing to HGSOC growth.
In addition, establishing the sequence of ovarian tumorigenesis and confirming the origin of HGSOC raise important questions regarding the clinical management of the disease. If some HGSOCs arise from tubal precursor lesions, how can one explain that the prolonged treatment with the combined oral contraceptive pill decreases the risk of OC? It may potentially reduce the incidence of cytotoxic stress during ovulation events or hormonal dysregulation potentially plays a role in ovarian tumorigenesis.
Another important clinical decision will be the usefulness of prophylactic or opportunistic surgical procedures, i.e., is there a clear incentive to perform a salpingectomy or a salpingo-oophorectomy to reduce the risk of HGSOC? On the one hand, clinicians should reconsider whether irreversible contraception should rather be performed by bilateral salpingectomy than tubal ligation if it reduces the risk of developing OC [53]. On the other hand, there is evidence of the beneficial effect of prophylactic bilateral salpingo-oophorectomy in patients with BRCA1/2 mutations, which should be performed at the age of 40–45 [53]. In this high-risk patient group, it was shown that prophylactic bilateral salpingo-oophorectomy significantly reduces the risk of developing both breast cancer and BRCA-related gynaecological cancer (hazard ratio 0.25, 95% CI 0.08–0.74) [62]. Despite the clear evidence in BRCA mutation carriers, the potential benefits of a bilateral salpingectomy are less clearly defined in patients without an increased genetic risk. Opportunistic bilateral salpingectomy is typically discussed with patients undergoing abdominal surgical procedures for other indications, such as hysterectomy. It remains to be seen whether prophylactic bilateral salpingectomy is an effective cancer prevention in women with low genetic risk. Interestingly, a recent multi-centre randomised controlled trial (n = 64) showed that opportunistic salpingectomy in patients undergoing laparoscopic hysterectomy did neither decrease ovarian reserve, measured as pre- and post-operative anti-müllerian hormone (p values > 0.2), nor increased surgical risk [63]. This is a relatively small trial, but it suggests that salpingectomy does not affect ovarian function while potentially reducing the risk of OC. This would have major clinical implications for the management of HGSOC and it will require carefully designed clinical trials, given the unclear benefit of opportunistic bilateral salpingectomy and the yet still uncertain origin of HGSOC [53].
References
Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A, Group AGOOCS (2007) Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol Oncol 106(1):69–74
Prat J (2012) New insights into ovarian cancer pathology. Ann Oncol 23(Suppl 10):x111–x117
Králíčková M, Vetvicka V (2014) Endometriosis and ovarian cancer. World J Clin Oncol 5(5):800–805
Young RH (2006) From krukenberg to today: the ever present problems posed by metastatic tumors in the ovary: part I. Historical perspective, general principles, mucinous tumors including the krukenberg tumor. Adv Anat Pathol 13(5):205–227
Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG (2017) The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer 17(1):65–74
Vang R, Shih IM, Kurman RJ (2009) Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol 16(5):267–282
Barnes B, Berta J, Buttmann-Schweiger N, Liebig J, Jordan S, Kramwinkel K, Niemann H, Nowossadeck E, Poethko-Müller C, Prytz F, Rattay P, Schönfeld I, Starker A, Wienecke A, Wolf U (2016) Bericht zum Krebsgeschehen in Deutschland 2016. Robert Koch Institut, Berlin
Wimberger P, Wehling M, Lehmann N, Kimmig R, Schmalfeldt B, Burges A, Harter P, Pfisterer J, du Bois A (2010) Influence of residual tumor on outcome in ovarian cancer patients with FIGO stage IV disease: an exploratory analysis of the AGO-OVAR (Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group). Ann Surg Oncol 17(6):1642–1648
Ayhan A, Kurman RJ, Yemelyanova A, Vang R, Logani S, Seidman JD, Shih IM (2009) Defining the cut point between low-grade and high-grade ovarian serous carcinomas: a clinicopathologic and molecular genetic analysis. Am J Surg Pathol 33(8):1220–1224
Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM (2012) High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA 109(10):3921–3926
Auersperg N (2013) The origin of ovarian cancers—hypotheses and controversies. Front Biosci (Schol Ed) 5:709–719
Moser AR, Pitot HC, Dove WF (1990) A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247(4940):322–324
Holme Ø, Bretthauer M, Fretheim A, Odgaard-Jensen J, Hoff G (2013) Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev 9:CD009259
Vaughan S, Coward JI, Bast RC, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM, Sood AK, Stronach EA, Walczak H, Bowtell DD, Balkwill FR (2011) Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 11(10):719–725
Auersperg N (2013) The origin of ovarian cancers—hypotheses and controversies. Front Biosci (Schol Ed) 5:709–719
Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM (2012) High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA 109(10):3921–3926
Fathalla MF (1971) Incessant ovulation—a factor in ovarian neoplasia? Lancet 2(7716):163
Ness RB, Grisso JA, Klapper J, Schlesselman JJ, Silberzweig S, Vergona R, Morgan M, Wheeler JE (2000) Risk of ovarian cancer in relation to estrogen and progestin dose and use characteristics of oral contraceptives. SHARE Study Group. Steroid hormones and reproductions. Am J Epidemiol 152(3):233–241
Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, Tang J, Li S, Zhang S, Shaw PA, Narod SA (2006) Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst 98(23):1694–1706
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130
Network CGAR (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474(7353):609–615
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W et al (1994) A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266(5182):66–71
Moynahan ME, Pierce AJ, Jasin M (2001) BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell 7(2):263–272
Moynahan ME, Chiu JW, Koller BH, Jasin M (1999) Brca1 controls homology-directed DNA repair. Mol Cell 4(4):511–518
Rebbeck TR, Mitra N, Wan F, Sinilnikova OM, Healey S, McGuffog L, Mazoyer S, Chenevix-Trench G, Easton DF, Antoniou AC, Nathanson KL, Laitman Y, Kushnir A, Paluch-Shimon S, Berger R, Zidan J, Friedman E, Ehrencrona H, Stenmark-Askmalm M, Einbeigi Z, Loman N, Harbst K, Rantala J, Melin B, Huo D, Olopade OI, Seldon J, Ganz PA, Nussbaum RL, Chan SB, Odunsi K, Gayther SA, Domchek SM, Arun BK, Lu KH, Mitchell G, Karlan BY, Walsh C, Lester J, Godwin AK, Pathak H, Ross E, Daly MB, Whittemore AS, John EM, Miron A, Terry MB, Chung WK, Goldgar DE, Buys SS, Janavicius R, Tihomirova L, Tung N, Dorfling CM, van Rensburg EJ, Steele L, Neuhausen SL, Ding YC, Ejlertsen B, Gerdes AM, Hansen T, Ramón y Cajal T, Osorio A, Benitez J, Godino J, Tejada MI, Duran M, Weitzel JN, Bobolis KA, Sand SR, Fontaine A, Savarese A, Pasini B, Peissel B, Bonanni B, Zaffaroni D, Vignolo-Lutati F, Scuvera G, Giannini G, Bernard L, Genuardi M, Radice P, Dolcetti R, Manoukian S, Pensotti V, Gismondi V, Yannoukakos D, Fostira F, Garber J, Torres D, Rashid MU, Hamann U, Peock S, Frost D, Platte R, Evans DG, Eeles R, Davidson R, Eccles D, Cole T, Cook J, Brewer C, Hodgson S, Morrison PJ, Walker L, Porteous ME, Kennedy MJ, Izatt L, Adlard J, Donaldson A, Ellis S, Sharma P, Schmutzler RK, Wappenschmidt B, Becker A, Rhiem K, Hahnen E, Engel C, Meindl A, Engert S, Ditsch N, Arnold N, Plendl HJ, Mundhenke C, Niederacher D, Fleisch M, Sutter C, Bartram CR, Dikow N, Wang-Gohrke S, Gadzicki D, Steinemann D, Kast K, Beer M, Varon-Mateeva R, Gehrig A, Weber BH, Stoppa-Lyonnet D, Houdayer C, Belotti M, Gauthier-Villars M, Damiola F, Boutry-Kryza N, Lasset C, Sobol H, Peyrat JP, Muller D, Fricker JP, Collonge-Rame MA, Mortemousque I, Nogues C, Rouleau E, Isaacs C, De Paepe A, Poppe B, Claes K, De Leeneer K, Piedmonte M, Rodriguez G, Wakely K, Boggess J, Blank SV, Basil J, Azodi M, Phillips KA, Caldes T, de la Hoya M, Romero A, Nevanlinna H, Aittomäki K, van der Hout AH, Hogervorst FB, Verhoef S, Collée JM, Seynaeve C, Oosterwijk JC, Gille JJ, Wijnen JT, Gómez Garcia EB, Kets CM, Ausems MG, Aalfs CM, Devilee P, Mensenkamp AR, Kwong A, Olah E, Papp J, Diez O, Lazaro C, Darder E, Blanco I, Salinas M, Jakubowska A, Lubinski J, Gronwald J, Jaworska-Bieniek K, Durda K, Sukiennicki G, Huzarski T, Byrski T, Cybulski C, Toloczko-Grabarek A, Złowocka-Perłowska E, Menkiszak J, Arason A, Barkardottir RB, Simard J, Laframboise R, Montagna M, Agata S, Alducci E, Peixoto A, Teixeira MR, Spurdle AB, Lee MH, Park SK, Kim SW, Friebel TM, Couch FJ, Lindor NM, Pankratz VS, Guidugli L, Wang X, Tischkowitz M, Foretova L, Vijai J, Offit K, Robson M, Rau-Murthy R, Kauff N, Fink-Retter A, Singer CF, Rappaport C, Gschwantler-Kaulich D, Pfeiler G, Tea MK, Berger A, Greene MH, Mai PL, Imyanitov EN, Toland AE, Senter L, Bojesen A, Pedersen IS, Skytte AB, Sunde L, Thomassen M, Moeller ST, Kruse TA, Jensen UB, Caligo MA, Aretini P, Teo SH, Selkirk CG, Hulick PJ, Andrulis I, Consortium C (2015) Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 313(13):1347–1361
Bowen NJ, Walker LD, Matyunina LV, Logani S, Totten KA, Benigno BB, McDonald JF (2009) Gene expression profiling supports the hypothesis that human ovarian surface epithelia are multipotent and capable of serving as ovarian cancer initiating cells. BMC Med Genom 2:71
Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H (2012) Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337(6095):730–735
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H (2009) Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457(7229):608–611
Pothuri B, Leitao MM, Levine DA, Viale A, Olshen AB, Arroyo C, Bogomolniy F, Olvera N, Lin O, Soslow RA, Robson ME, Offit K, Barakat RR, Boyd J (2010) Genetic analysis of the early natural history of epithelial ovarian carcinoma. PLoS One 5(4):e10358
Sharma A, Gentry-Maharaj A, Burnell M, Fourkala EO, Campbell S, Amso N, Seif MW, Ryan A, Parmar M, Jacobs I, Menon U (2012) Assessing the malignant potential of ovarian inclusion cysts in postmenopausal women within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a prospective cohort study. BJOG 119(2):207–219
Dubeau L (1999) The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gynecol Oncol 72(3):437–442
Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP (2007) Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol 31(2):161–169
Kurman RJ, Shih IM (2011) Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—shifting the paradigm. Hum Pathol 42(7):918–931
Zeppernick F, Meinhold-Heerlein I, Shih IM (2015) Precursors of ovarian cancer in the fallopian tube: serous tubal intraepithelial carcinoma—an update. J Obstet Gynaecol Res 41(1):6–11
Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH (2001) Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol 195(4):451–456
Piek JM, Kenemans P, Verheijen RH (2004) Intraperitoneal serous adenocarcinoma: a critical appraisal of three hypotheses on its cause. Am J Obstet Gynecol 191(3):718–732
Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R, Stewart C, Fereday S, Caldas C, Defazio A, Bowtell D, Brenton JD (2010) Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol 221(1):49–56
Singer G, Stohr R, Cope L, Dehari R, Hartmann A, Cao DF, Wang TL, Kurman RJ, Shih Ie M (2005) Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis: a mutational analysis with immunohistochemical correlation. Am J Surg Pathol 29(2):218–224
Hofstetter G, Berger A, Schuster E, Wolf A, Hager G, Vergote I, Cadron I, Sehouli J, Braicu EI, Mahner S, Speiser P, Marth C, Zeimet AG, Ulmer H, Zeillinger R, Concin N (2011) Delta133p53 is an independent prognostic marker in p53 mutant advanced serous ovarian cancer. Br J Cancer 105(10):1593–1599
Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP (2007) A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol 211(1):26–35
Rebbeck TR, Kauff ND, Domchek SM (2009) Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst 101(2):80–87
Kuhlmann JD, Baraniskin A, Hahn SA, Mosel F, Bredemeier M, Wimberger P, Kimmig R, Kasimir-Bauer S (2014) Circulating U2 small nuclear RNA fragments as a novel diagnostic tool for patients with epithelial ovarian cancer. Clin Chem 60(1):206–213
Wimberger P, Chebouti I, Kasimir-Bauer S, Lachmann R, Kuhlisch E, Kimmig R, Süleyman E, Kuhlmann JD (2014) Explorative investigation of vascular endothelial growth factor receptor expression in primary ovarian cancer and its clinical relevance. Gynecol Oncol 133(3):467–472
Barker N, Bartfeld S, Clevers H (2010) Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell 7(6):656–670
Ng A, Tan S, Singh G, Rizk P, Swathi Y, Tan TZ, Huang RY, Leushacke M, Barker N (2014) Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat Cell Biol 16(8):745–757
Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, Ootani A, Roelf K, Lee M, Yuan J, Li X, Bolen CR, Wilhelmy J, Davies PS, Ueno H, von Furstenberg RJ, Belgrader P, Ziraldo SB, Ordonez H, Henning SJ, Wong MH, Snyder MP, Weissman IL, Hsueh AJ, Mikkelsen TS, Garcia KC, Kuo CJ (2017) Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature 545(7653):238–242
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449(7165):1003–1007
Leushacke M, Barker N (2012) Lgr5 and Lgr6 as markers to study adult stem cell roles in self-renewal and cancer. Oncogene 31(25):3009–3022
Sun Y, Jia X, Wu X (2016) High Expressions of Lgr5 and ALDH1 in primary epithelial ovarian cancer correlate with advanced tumor stage and grade as well as poor prognosis of the patients. Gynecol Obstet Invest 81:162–168
Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY (2013) Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature 495(7440):241–245
Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, Berger H, Mollenkopf HJ, Mangler M, Sehouli J, Fotopoulou C, Meyer TF (2015) The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun 6:8989
Schmeler KM, Lu KH (2008) Gynecologic cancers associated with Lynch syndrome/HNPCC. Clin Transl Oncol 10(6):313–317
Pölcher M, Hauptmann S, Fotopoulou C, Schmalfeldt B, Meinhold-Heerlein I, Mustea A, Runnebaum I, Sehouli J (2015) Opportunistic salpingectomies for the prevention of a high-grade serous carcinoma: a statement by the Kommission Ovar of the AGO. Arch Gynecol Obstet 292(1):231–234
Kotsopoulos J, Terry KL, Poole EM, Rosner B, Murphy MA, Hecht JL, Crum CP, Missmer SA, Cramer DW, Tworoger SS (2013) Ovarian cancer risk factors by tumor dominance, a surrogate for cell of origin. Int J Cancer 133(3):730–739
Mungenast F, Thalhammer T (2014) Estrogen biosynthesis and action in ovarian cancer. Front Endocrinol (Lausanne) 5:192
Zhou B, Sun Q, Cong R, Gu H, Tang N, Yang L, Wang B (2008) Hormone replacement therapy and ovarian cancer risk: a meta-analysis. Gynecol Oncol 108(3):641–651
Hernandez E, Rosenshein NB, Bhagavan BS, Parmley TH (1984) Tumor heterogeneity and histopathology in epithelial ovarian cancer. Obstet Gynecol 63(3):330–334
Alizadeh AA, Aranda V, Bardelli A, Blanpain C, Bock C, Borowski C, Caldas C, Califano A, Doherty M, Elsner M, Esteller M, Fitzgerald R, Korbel JO, Lichter P, Mason CE, Navin N, Pe’er D, Polyak K, Roberts CW, Siu L, Snyder A, Stower H, Swanton C, Verhaak RG, Zenklusen JC, Zuber J, Zucman-Rossi J (2015) Toward understanding and exploiting tumor heterogeneity. Nat Med 21(8):846–853
Merritt MA, Bentink S, Schwede M, Iwanicki MP, Quackenbush J, Woo T, Agoston ES, Reinhardt F, Crum CP, Berkowitz RS, Mok SC, Witt AE, Jones MA, Wang B, Ince TA (2013) Gene expression signature of normal cell-of-origin predicts ovarian tumor outcomes. PLoS One 8(11):e80314
Domcke S, Sinha R, Levine DA, Sander C, Schultz N (2013) Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun 4:2126
Ince TA, Sousa AD, Jones MA, Harrell JC, Agoston ES, Krohn M, Selfors LM, Liu W, Chen K, Yong M, Buchwald P, Wang B, Hale KS, Cohick E, Sergent P, Witt A, Kozhekbaeva Z, Gao S, Agoston AT, Merritt MA, Foster R, Rueda BR, Crum CP, Brugge JS, Mills GB (2015) Characterization of twenty-five ovarian tumour cell lines that phenocopy primary tumours. Nat Commun 6:7419
Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, Ellis NA, Boyd J, Borgen PI, Barakat RR, Norton L, Castiel M, Nafa K, Offit K (2002) Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 346(21):1609–1615
Song T, Kim MK, Kim ML, Jung YW, Yun BS, Seong SJ, Kwon SH (2017) Impact of opportunistic salpingectomy on anti-Müllerian hormone in patients undergoing laparoscopic hysterectomy: a multicentre randomised controlled trial. BJOG 124(2):314–320
Author information
Authors and Affiliations
Contributions
DMK: project development, data collection, manuscript writing, and editing. PW: supervision and manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Klotz, D.M., Wimberger, P. Cells of origin of ovarian cancer: ovarian surface epithelium or fallopian tube?. Arch Gynecol Obstet 296, 1055–1062 (2017). https://doi.org/10.1007/s00404-017-4529-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4529-z