Abstract
Purpose
The aim of this study was to evaluate the role of maternal serum total Homocysteine (tHcy) and uterine artery (Ut-A) Doppler as predictors of preeclampsia (PE), intrauterine growth restriction (IUGR), and other complications related to poor placentation.
Patients and methods
A prospective cohort study was conducted on 500 women with spontaneous pregnancies. tHcy was measured at 15–19 weeks, and then, Ut-A Doppler was performed at 18–22 weeks of pregnancy.
Results
453 pregnant women completed the follow-up of the study. The tHcy and Ut-A resistance index were significantly higher in women who developed PE, IUGR, and other complications when compared to controls (tHcy: 7.033 ± 2.744, 6.321 ± 3.645, and 6.602 ± 2.469 vs 4.701 ± 2.082 μmol/L, respectively, p value <0.001 and Ut-A resistance index: 0.587 ± 0.072, 0.587 ± 0.053, and 0.597 ± 0.069 vs 0.524 ± 0.025, respectively, p value <0.001). The use of both tHcy assessment and Ut-A Doppler improved the sensitivity of prediction of PE relative to the use of each one alone (85.2 relative to 73.33 and 60%, respectively).
Conclusion
The use of elevated homocysteine and uterine artery Doppler screening are valuable in prediction of preeclampsia, IUGR, and poor placentation disorders.
ClincalTrial.gov ID
NCT02854501.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Placentation is a complex process initiated in the first trimester of pregnancy and correct vascular adaptation between maternal and fetal circulations is necessary for establishment of adequate placental growth [1]. To reduce the vessel resistance and to increase uteroplacental blood flow, the spiral arteries undergo a series of vascular transformations through two waves of trophoblastic invasion at 11–14 and 20–24 weeks of gestation [2]. Uterine artery mean flow velocity normally increases nearly eight times from non-pregnant state (8.4 cm/s) to 36 weeks of pregnancy (61.4 cm/s) [3].
The underlying cause of preeclampsia (PE) is believed to be poor placentation [4]. The hypothesized factors leading to abnormal trophoblastic invasions are disruptions of endothelial junctional proteins [5], subendothelial changes in uterine arteries [6], and generalized vascular spasms [7].
Homocysteine is an amino acid that is closely linked to folate metabolism. Its level declines during normal pregnancy [8]. Elevated total homocysteine (tHcy) levels have been shown to be deleterious on vascular endothelium [9, 10]. The abnormal high level during pregnancy has been linked to PE [8], abortions [11, 12], low birth weight [8], and increased incidence of type 2 diabetes in the offspring [13].
The prediction of the obstetric complications that are related to abnormal placentation before they become clinically evident is an important aim in the antenatal screening of low-risk populations. Hence, indicators of increased resistances in the placental vascular bed due to impaired placentation such as elevated tHcy level and abnormal uterine artery (Ut-A) Doppler velocimetry may be used in the screening of low-risk population [8, 14,15,16,17,18]. Analysis of uterine arteries Doppler waveform helps in assessment of blood flow at the maternofetal level. From the 24th week of pregnancy, persistence of high resistance in the uterine arteries may reflect the failure of normal placentation and the wave may have a diastolic notch [1].
The aim of our study was to evaluate the role of maternal serum total homocysteine and uterine artery Doppler as predictors of preeclampsia and poor placentation disorders.
Materials and methods
This prospective cohort study was conducted at Kasr El Aini Hospital, Cairo University, Egypt, in the period from September 2015 to August 2016. Informed written consent was obtained from all participants. The study was approved by the local ethics committee.
500 women with spontaneous pregnancies without history of previous risk factors were enrolled in the current study. None of our patients had history of PE. Exclusion criteria included multiple pregnancy, age above 40 years, impaired renal function, and hypertensive disorder before 20 weeks of gestation. Patients having diabetes or other chronic diseases were excluded. Women taking folic acid supplementations or antifolate drugs were also excluded from the study.
During the first antenatal care visit, participants were questioned about age, parity, past and family history of chronic diseases, and smoking status. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Systolic and diastolic blood pressure readings were recorded. Gestational age (GA) was calculated using the date of last menstrual period and confirmed by first trimesteric ultrasound.
Samples of the tHcy measurement were collected between 15 and 19 week GA. Venous blood samples were taken after overnight fasting for serum homocysteine estimation. The serum was separated by centrifugation. Assay for homocysteine by chemiluminescence technique was performed using a calibrated fully automated system; Advia Centaur CP immunoassay system (Siemens Healthcare, Erlangen, Germany) [19].
All participants had uterine artery Doppler investigations, as part of a routine scan between the 18th and 22nd weeks of pregnancy. Uterine artery Doppler velocimetry was performed with the Medison X6 ultrasound scanner (Medison, Seoul, South Korea) at the crossing of the uterine and external iliac arteries with an insonation angle of less than 30° and a velocity of more than 60 cm/s. The sample volume was set at 2.0 mm. Three measurements were taken on each side and the averages were calculated [7]. One operator performed the Doppler measurements for consistency. The use of uterine artery resistance index (Ut-A RI) cut-off points and bilateral or unilateral notches has already been shown to improve the efficacy of uterine artery Doppler screening for complications related to uterplacental insufficiency [20].
Follow-up for all pregnant women was done every 2–4 weeks until delivery according to the hospital protocol in each case. During these follow-up visits, measuring of blood pressure with or without ultrasound was done. The ultrasound examination was performed for confirmation of fetal viability, estimation of fetal weight, assessment of amount of liquor and measuring gestational age through assessment of the biparietal diameter, head circumference, abdominal circumference, and femur length.
The participants were then divided into four groups according to the outcome: the first group (group 1) did not develop any of the complications, and the second group (group 2) were pregnant women who developed preeclampsia. The third group (group 3) of participants were those who had IUGR, while the fourth group (group 4) were participants who developed other complications (abruptio placentae or stillbirth). Preeclampsia was diagnosed as: the development of hypertension (persistent systolic blood pressure of 140 mmHg or higher, or a diastolic blood pressure of 90 mmHg or higher) after 20 weeks of gestation in a woman with previously normal blood pressure and proteinuria (greater than or equals 300 mg per 24 h urine). New onset hypertension in the absence of proteinuria but combined with other complications as renal insufficiency, thrombocytopenia, renal insufficiency, liver impairment, pulmonary edema, or cerebral or visual symptoms was also diagnostic of PE [21]. IUGR was defined as delivery of a living infant with a birth weight below the tenth centile for gestation [22]. Abruptio placentae was diagnosed when the separation of the normally implanted placenta resulted in concealed or revealed antepartum hemorrhage [23].
Sample size calculation was based on the sensitivity of abnormal Doppler and homocysteine level in predicting pregnancy complications. Prior data indicated that the average sensitivity of abnormal Doppler and homocysteine level in predicting pregnancy complications was 61% and the incidence of PE, IUGR, and other complications ranged from 3 to 15% taking 10% as average [24]. Setting the type I error probability at 0.05 and power at 80%, 430 participants were needed. Considering 10% drop rate so we started with 500 participants. Calculations were done using Flahault and associates tables [25].
Data were statistically described in terms of mean ± standard deviation (±SD), median and range, or frequencies (number of cases) and percentages when appropriate. Comparison of means between the study groups was done using one-way analysis of variance (ANOVA) test with posthoc multiple two-group comparisons after adjustment for alpha error. Kruskal–Wallis test was used for comparing medians between groups. For comparing categorical data, Chi-square (χ 2) test was performed. Exact test was used instead when the expected frequency is less than 5. Receiver operator characteristic (ROC) analysis was used to determine the optimum cut-off value for the studied tHcy and Ut-A RI for prediction of poor placentation disorders. p value less than 0.05 was considered statistically significant. All statistical calculations were done by the SPSS software, version 23 (IBM Corp., Armonk, NY, USA).
Results
Flow chart of the study population is presented in Fig. 1. 453 pregnant women completed the follow-up until delivery and were included in the analysis of data.
There was no significant difference between all study groups regarding age, parity, and body mass index (Table 1). The GA at sampling for tHcy was statistically slightly higher (only 0.5 week difference) in the IUGR group relative to other groups; however, this was of no clinical importance (Table 2). There was a significant difference between women who developed PE and IUGR when compared to uncomplicated group regarding gestational age at delivery (37 ± 1.52 and 37.35 ± 1.47 vs 38.84 ± 1.98 weeks, respectively, p value <0.001). The percentages of smokers were significantly higher in the IUGR and other complications groups relative to the uncomplicated and PE groups (34.6 and 32 vs 14.1 and 11%, respectively, p value 0.003) (Tables 1, 2). The neonatal birth weight was significantly lower in women who developed PE, IUGR, and other complications when compared to uncomplicated group (2793.33 ± 247.4, 2466.54 ± 306.1, and 2993.76 ± 252.06 vs 3277.07 ± 243.06 g, respectively, p value <0.001), while the occurrence of intrauterine fetal death was higher in PE, IUGR, and other complications groups relative to uncomplicated group (6.7, 11.5, and 11.8 vs 0%, respectively, p value 0.001). The percentage of preterm birth was higher in the PE, IUGR, and other complications groups when compared to the uncomplicated groups (26.7, 21.2, and 20.34 vs 8%, respectively; p value <0.001) (Tables 1, 2).
The tHcy and Ut-A RI were significantly higher in PE, IUGR, and other complications groups when compared to the uncomplicated group (for tHcy: 7.033 ± 2.744, 6.321 ± 3.645, and 6.602 ± 2.469 vs 4.701 ± 2.082 μmol/L, respectively, p value <0.001 and for Ut-A RI: 0.587 ± 0.072, 0.587 ± 0.053, and 0.597 ± 0.069 vs. 0.524 ± 0.025, respectively) (Tables 1, 2).
The percentages of participants who had uterine artery notch (unilateral or bilateral) were significantly higher in PE, IUGR, and other complications groups when compared to the uncomplicated group (Tables 1, 2).
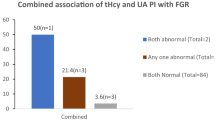
The sensitivity, specificity, and other accuracy measures of plasma total homocysteine and uterine artery RI for prediction for PE, IUGR, and other complications are shown in Table 3 and Figs. 2 and 3. The combined use of abnormal uterine artery Doppler and tHcy cutoffs improved the accuracy in prediction of PE relative to the use of each predictor alone (for the combined predictors: sensitivity: 85.2%, specificity: 89.9%, positive predictive value, PPV: 83.6%, negative predictive value, and NPV: 91.2%). Improved accuracy measures were observed also for prediction of IUGR and other complications groups (Table 3; Fig. 4).
Discussion
The current prospective study investigated the role of tHcy and Ut-A Doppler in prediction of complications related to impaired placentation, such as preeclampsia and IUGR.
There was a significant difference between women who developed PE, IUGR, and other complications when compared to controls regarding homocysteine level measured between 15 and 19 weeks of gestation and uterine artery resistance index done at 18–22 weeks of gestation. The combined use of tHcy and Ut-A RI improved the sensitivity for prediction of preeclampsia (85.2 vs 73.3% for tHcy alone and 60% for Ut-A RI). Other accuracy measures were also improved for prediction of preeclampsia and other abnormal placentation disorders with the use of both predictors.
Increased tHcy is an established risk factor for endothelial dysfunction. Elevated plasma homocysteine was suggested as a risk factor for vascular diseases, such as atherosclerosis and occlusive vascular disorders, and it has also been associated with some pregnancy complications, such as neural tube defects, repeated miscarriages [26], abruptio placentae, fetal death, preeclampsia, and IUGR [27, 28]. Vitamins B6, B9, and B12 and folic acid are needed as cofactors for proper function of the enzymes in homocysteine cycle. Thus, nutritional deficiency of these vitamins can lead to increase in blood tHcy levels. Dietary supplementation for such vitamins has been suggested to treat elevation of plasma homocysteine [29].
There are similar studies investigating the association between uterine artery Doppler velocimetry, elevated homocysteine concentration with abnormal placentation. Lopez and co-workers studied 94 low-risk pregnant women. They measured tHcy, folate, and Vitamin B12 and assessed the uterine Doppler velocimetry. They concluded that there was a value for the addition of measuring plasma homocysteine to Doppler data in prediction of pregnancy complications [1]. Onalan and colleagues evaluated the value of a combined second trimesteric maternal serum homocysteine and uterine artery Doppler screening in prediction of preeclampsia, intrauterine growth restriction (IUGR), abruptio placentae, and stillbirth. They found that the sensitivity for prediction of preeclampsia using the combined method was 61.3% [24]. Another cohort study was performed on 100 antenatal women between 8 and 12 weeks of gestation. The investigators found significant correlation between first trimestric elevated homocysteine level and some adverse maternal and perinatal outcomes as miscarriage and preeclampsia [30].
In contrast to our study, Kaymaz and associates did not find that the addition of assessment of homocysteine level increased the sensitivity in prediction of preeclampsia [2]. However, only 103 pregnant women were studied and few pregnant women developed complications.
To the best of our knowledge, this is the first prospective study with a large sample size that was done to evaluate the combined accuracy homocysteine and uterine artery Doppler in prediction of abnormal placentation disorders.
The main limitation of our study is the inability to continue follow-up for neonatal outcome including neonatal ICU admission until discharge or follow-up development of maternal complications.
Conclusion
The use of elevated homocysteine and uterine artery Doppler screening is valuable in prediction of preeclampsia, IUGR, and poor placentation disorders. The combined use of uterine artery doppler velocimetry and serum total homocysteine improved the accuracy of prediction of poor placentation disorders more than each predictor alone.
Change history
19 March 2024
An Editorial Expression of Concern to this paper has been published: https://doi.org/10.1007/s00404-024-07454-w
15 April 2024
An Editorial Expression of Concern to this paper has been published: https://doi.org/10.1007/s00404-024-07491-5
References
Lopez-Quesada E, Vilaseca MA, Vela A, Lailla JM (2004) Perinatal outcome prediction by maternal homocysteine and uterine artery Doppler velocimetry. Eur J Obstet Gynecol Reprod Biol 113(1):61–66. doi:10.1016/j.ejogrb.2003.05.003
Kaymaz C, DemirA Bige O, Cagliyan E, Cimrin D, Demir N (2011) Analysis of perinatal outcome by combination of first trimester maternal plasma homocysteine with uterine artery Doppler velocimetry. Prenat Diagn 31(13):1246–1250. doi:10.1002/pd.2874
Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG (1992) Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 80(6):1000–1006
Roberts JM, Cooper DW (2001) Pathogenesis and genetics of pre-eclampsia. Lancet 357(9249):53–56
Wang Y, Gu Y, Granger DN, Roberts JM, Alexander JS (2002) Endothelial junctional protein redistribution and increased monolayer permeability in human umbilical vein endothelial cells isolated during preeclampsia. Am J Obstet Gynecol 186(2):214–220
Suzuki Y, Yamamoto T, Mabuchi Y, Tada T, Suzumori K, Soji T, Herbert DC, Itoh T (2003) Ultrastructural changes in omental resistance artery in women with preeclampsia. Am J Obstet Gynecol 189(1):216–221
Maged AM, ElNassery N, Fouad M, Abdelhafiz A, Al Mostafa W (2015) Third-trimester uterine artery Doppler measurement and maternal postpartum outcome among patients with severe pre-eclampsia. Int J Gynaecol Obstetr 131(1):49–53. doi:10.1016/j.ijgo.2015.03.045
Dodds L, Fell DB, Dooley KC, Armson BA, Allen AC, Nassar BA, Perkins S, Joseph KS (2008) Effect of homocysteine concentration in early pregnancy on gestational hypertensive disorders and other pregnancy outcomes. Clin Chem 54(2):326–334. doi:10.1373/clinchem.2007.097469
Yang Y, Luo Y, Yuan J, Tang Y, Xiong L, Xu M, Rao X, Liu H (2016) Association between maternal, fetal and paternal MTHFR gene C677T and A1298C polymorphisms and risk of recurrent pregnancy loss: a comprehensive evaluation. Arch Gynecol Obstet 293(6):1197–1211. doi:10.1007/s00404-015-3944-2
Kramer MS, Kahn SR, Rozen R, Evans R, Platt RW, Chen MF, Goulet L, Seguin L, Dassa C, Lydon J, McNamara H, Dahhou M, Genest J (2009) Vasculopathic and thrombophilic risk factors for spontaneous preterm birth. Int J Epidemiol 38(3):715–723. doi:10.1093/ije/dyp167
Yin Y, Zhang T, Dai Y, Zheng X, Pei L, Lu X (2009) Pilot study of association of anembryonic pregnancy with 55 elements in the urine, and serum level of folate, homocysteine and S-adenosylhomocysteine in Shanxi Province, China. J Am Coll Nutr 28(1):50–55
Chen H, Yang X, Lu M (2016) Methylenetetrahydrofolate reductase gene polymorphisms and recurrent pregnancy loss in China: a systematic review and meta-analysis. Arch Gynecol Obstet 293(2):283–290. doi:10.1007/s00404-015-3894-8
Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, Bhat DS, Naik SS, Coyaji KJ, Joglekar CV, Joshi N, Lubree HG, Deshpande VU, Rege SS, Fall CH (2008) Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 51(1):29–38. doi:10.1007/s00125-007-0793-y
Caforio L, Testa AC, Mastromarino C, Carducci B, Ciampelli M, Mansueto D, Caruso A (1999) Predictive value of uterine artery velocimetry at midgestation in low- and high-risk populations: a new perspective. Fetal Diagn Ther 14(4):201–205. doi:10.1159/000020921
Zamarian ACP, Araujo Júnior E, Daher S, Rolo LC, Moron AF, Nardozza LMM (2016) Evaluation of biochemical markers combined with uterine artery Doppler parameters in fetuses with growth restriction: a case–control study. Arch Gynecol Obstet 294(4):715–723. doi:10.1007/s00404-016-4024-y
Stubert J, Kleber T, Bolz M, Külz T, Dieterich M, Richter D-U, Reimer T (2016) Acute-phase proteins in prediction of preeclampsia in patients with abnormal midtrimester uterine Doppler velocimetry. Arch Gynecol Obstet 294(6):1151–1160. doi:10.1007/s00404-016-4138-2
Noguchi J, Tanaka H, Koyanagi A, Miyake K, Hata T (2015) Three-dimensional power Doppler indices at 18–22 weeks’ gestation for prediction of fetal growth restriction or pregnancy-induced hypertension. Arch Gynecol Obstet 292(1):75–79. doi:10.1007/s00404-014-3603-z
Uyar İ, Kurt S, Demirtaş Ö, Gurbuz T, Aldemir OS, Keser B, Tasyurt A (2015) The value of uterine artery Doppler and NT-proBNP levels in the second trimester to predict preeclampsia. Arch Gynecol Obstet 291(6):1253–1258. doi:10.1007/s00404-014-3563-3
Ducros V, Demuth K, Sauvant MP, Quillard M, Causse E, Candito M, Read MH, Drai J, Garcia I, Gerhardt MF (2002) Methods for homocysteine analysis and biological relevance of the results. J Chromatogr, B: Anal Technol Biomed Life Sci 781(1–2):207–226
Kurdi W, Campbell S, Aquilina J, England P, Harrington K (1998) The role of color Doppler imaging of the uterine arteries at 20 weeks’ gestation in stratifying antenatal care. Ultrasound Obstetr Gynecol 12(5):339–345. doi:10.1046/j.1469-0705.1998.12050339.x
Snydal S (2014) Major changes in diagnosis and management of preeclampsia. J Midwifery Women’s Health 59(6):596–605. doi:10.1111/jmwh.12260
Unterscheider J, O’Donoghue K, Malone FD (2015) Guidelines on fetal growth restriction: a comparison of recent national publications. Am J Perinatol 32(4):307–316. doi:10.1055/s-0034-1387927
Tikkanen M (2011) Placental abruption: epidemiology, risk factors and consequences. Actaobstetricia et gynecologicaScandinavica 90(2):140–149. doi:10.1111/j.1600-0412.2010.01030.x
Onalan R, Onalan G, Gunenc Z, Karabulut E (2006) Combining 2nd-trimester maternal serum homocysteine levels and uterine artery Doppler for prediction of preeclampsia and isolated intrauterine growth restriction. Gynecol Obstet Invest 61(3):142–148. doi:10.1159/000090432
Flahault A, Cadilhac M, Thomas G (2005) Sample size calculation should be performed for design accuracy in diagnostic test studies. J Clin Epidemiol 58(8):859–862. doi:10.1016/j.jclinepi.2004.12.009
Quere I, Bellet H, Hoffet M, Janbon C, Mares P, Gris JC (1998) A woman with five consecutive fetal deaths: case report and retrospective analysis of hyperhomocysteinemia prevalence in 100 consecutive women with recurrent miscarriages. Fertil Steril 69(1):152–154
Lopez-Quesada EL, Vilaseca MA, Gonzalez S (2000) Homocysteine and pregnancy. Medicinaclinica 115(9):352–356
Mignini LE, Latthe PM, Villar J, Kilby MD, Carroli G, Khan KS (2005) Mapping the theories of preeclampsia: the role of homocysteine. Obstet Gynecol 105(2):411–425. doi:10.1097/01.AOG.0000151117.52952.b6
Moat SJ, McDowell IF (2005) Homocysteine and endothelial function in human studies. Sem Vasc Med 5(2):172–182. doi:10.1055/s-2005-872402
Mascarenhas M, Habeebullah S, Sridhar MG (2014) Revisiting the role of first trimester homocysteine as an index of maternal and fetal outcome. J Pregnancy 2014:123024. doi:10.1155/2014/123024
Author information
Authors and Affiliations
Contributions
AMM: project development, data collection, and manuscript writing; HS: data collection; HM: data collection; ES: data collection; SA: manuscript writing and data collection; EO: data collection, data analysis, and manuscript writing; WD: manuscript writing; MK: manuscript writing.
Corresponding author
Ethics declarations
Ethical standards
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The author reports no conflicts of interest in this work.
Rights and permissions
About this article
Cite this article
Maged, A.M., Saad, H., Meshaal, H. et al. Maternal serum homocysteine and uterine artery Doppler as predictors of preeclampsia and poor placentation. Arch Gynecol Obstet 296, 475–482 (2017). https://doi.org/10.1007/s00404-017-4457-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4457-y