Abstract
Purpose
In general, male and female are prescribed the same amount of dosage even if most of the cases female required less dosage than male. Physicians are often facing problem on appropriate drug dosing, efficient treatment, and drug safety for a female in general. To identify and synthesize evidence about the effectiveness of gender-based therapy; provide the information to patients, providers, and health system intervention to ensure safety treatment; and minimize adverse effects.
Methods
We performed a systematic review to evaluate the effect of gender difference on pharmacotherapy. Published articles from January 1990 to December 2015 were identified using specific term in MEDLINE (PubMed), EMBASE, and the Cochrane library according to search strategies that strengthen the reporting of observational and clinical studies.
Results
Twenty-six studies fulfilled the inclusion criteria for this systematic review, yielding a total of 6309 subjects. We observed that female generally has a lower the gastric emptying time, gastric PH, lean body mass, and higher plasma volume, BMI, body fat, as well as reduce hepatic clearance, difference in activity of Cytochrome P450 enzyme, and metabolize drugs at different rate compared with male. Other significant factors such as conjugation, protein binding, absorption, and the renal elimination could not be ignored. However, these differences can lead to adverse effects in female especially for the pregnant, post-menopausal, and elderly women.
Conclusion
This systematic review provides an evidence for the effectiveness of dosage difference to ensure safety and efficient treatment. Future studies on the current topic are, therefore, recommended to reduce the adverse effect of therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the role of gender as a factor in drug pharmacokinetics and pharmacodynamics has become better appreciated [1]. Therefore, gender difference is a major area of interest within the field of drugs pharmacology. The usual weight difference between men and women can potentially influence body water spaces, muscle mass, organ blood flow, and organ function, and therefore, it could also affect pharmacokinetic parameters of many drugs, e.g., aliskirin, an antihypertensive rennin inhibitor, as well as fluconazole, an antifungal drug [2, 3]. Furthermore, women tend to have a higher percentage of body fat than men do which could affect the volume of distribution of lipophilic drugs [4] such as trazodone [5] and sufentanil [6], and many more. Women often exhibit a moderately faster clearance of drugs metabolized by the major metabolic CYP3A4 pathway [7] and also show alterations in the disposition of drugs in relation to the phase of the menstrual cycle [8], pregnancy [9], or after menopause [10].
Pharmacokinetic and pharmacodynamical changes can affect both the desired therapeutic effect of a drug as well as its adverse effect profile [11]. Assessment of pharmacodynamical differences between men and women requires the control of pharmacokinetic factors and should use the appropriate methodology to relate the response to a drug’s plasma and bio-phase concentrations [12]. There are many notable examples of marked gender differences in a drug’s effectiveness and efficacy. Aspirin is less effective in women in the prevention of stroke, which may be related to the gender hormone-dependent difference in platelets aggregation [13]. Pentazocaine, an opioid drug, shows greater efficacy for pain relief in women than in men, but ibuprofen exhibits a reverse response with no gender-associated differences in kinetics [14]. Corticosteroids drugs which are widely used for their anti-inflammatory and immunosuppressive properties also exhibit pharmacokinetics/pharmacodynamics which can be influenced by gender difference [12]. These findings suggest that gender-specific differences in body composition may result in variable drugs disposition and responsiveness.
Therefore, this paper provides a brief overview of the existing evidence for gender-specific differences in pharmacotherapy. This overview is organized along the following questions: (1) What is the fundamental difference between the male and female body composition? (2) What factors are responsible for a drug’s differential pharmacokinetics and pharmacodynamics? (3) Why it is the right time for gender-based pharmacotherapy? (4) What efforts are/research is needed to move forward? (5) What impact might gender based effect have for transforming the population’s health status? We systematically reviewed the data for gender-based differences in pharmacotherapy with the aim to provide a comprehensive review of this topic.
Method
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [15].
Data sources
A comprehensive search of MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials and Database of Systematic Reviews from the earliest available online year of indexing up to May 2016 was conducted. We used the following text words as search terms “gender difference pharmacokinetics/pharmacodynamics”, “sex difference pharmacokinetics/pharmacodynamics”, “male and female pharmacokinetics/pharmacodynamics”, “gender effect of medication”, “male and female difference”, etc. Our search included articles published in English and non-English languages. We also scanned the bibliographies of all retrieved articles for additional relevant articles. Further, the authors of potentially eligible abstracts, posters, or manuscripts were contacted via e-mail to obtain additional data, if possible. However, we did not include any unpublished data in our analysis.
Study selection and eligible criteria
Two of our authors (Md. M.I. and P.-A.N.) independently performed article selection, data extraction, and assessment of risk of bias. All disagreements were resolved by consensus with our main investigators.
Studies were included if they met the following inclusion criteria: (1) Controlled observational and clinical studies, (2) studies reported cases of male and female dose difference; (3) studies which had more than 5 participants, and (4) p value <0.05, or sufficient data were available to calculate a p value.
We excluded studies if for the following reasons: (1) it was only a case report, an editorial, or a review; or (2) studies that did not provide a sufficient amount of information regarding gender-specific difference in the pharmacokinetics and pharmacodynamics outcome.
Data extraction
Twenty-six studies that fulfilled all of our criteria as stated above were then independently entered into our database by the two authors, with the following entries: first author’s last name, publication year, country source of study, participants’ characteristics, method of ascertainment of dose difference, sample size, variable adjustment, etc. We also screened the title, abstract, and full text in a similar fashion; however, specific exclusion reasons were documented only during full-text screening. Upon selection of the final group of studies, the same two authors independently extracted the qualitative and quantitative data using a standardized data extraction form adjudicated by a third author (S.-A.S)
Outcome parameters
The two primary outcome parameters of this systematic review were: (1) to address the factors for gender-specific differences in pharmacokinetics and pharmacodynamics; and (2) to identify the medications which should have different dosage recommendations for men and women.
Results
Study selection
The search strategy identified 18,183 articles. Of these, 18,032 articles were excluded based on our predetermined eligibility criteria described above, while the remaining 151 articles underwent detailed full-text evaluation. Among these, only 26 published articles met our inclusion criteria. The most common reason for exclusion of the 125 excluded studies was lack of participants (n = 55), followed by ineligible study design (n = 37), unable to locate full text (n = 12), full-text duplication (n = 4), and so on. Figure 1 summarizes our selection process.
Study characteristics
This systematic review identified 6309 subjects which were mentioned in 21 studies; we also included five further studies which did not mention any gender-specific information. Table 1 shows the general characteristics of these 26 observational or clinical studies which we included in the final systematic review. Four studies were clinical trials, and 22 studies were observational studies. These studies were published between 1990 and 2016, spanning 26 years. 14 studies were conducted in North America, eight studies in Asia, and four studies in Europe. All studies included a significant number of subjects ranging from 14 to 1005.
Systematic review:
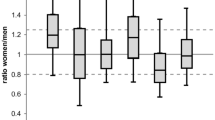
We conducted a primary systematic analysis using the 26 studies which reported results on 26 different groups of drugs based on gender-specific difference effect. A qualitative synthesis of these 26 studies is shown in Table 1. 17 out of 26 studies reported that a higher plasma drugs concentration for women, even though the dose was similar [16–32], and the range of drug plasma concentration in women was 10–30% higher than in men. However, in some cases, the drug plasma concentration in women was greater than 80% when compared to that of men [21, 31, 32]. We also found a slower clearance rate [25, 33–38] and a lower volume of distribution [12, 20, 33] in women compared to men. Only one study mentioned a faster clearance rate for women [39].
To further elicit the factors that are responsible for altering drugs pharmacokinetics and pharmacodynamics, we divided the drugs into different groups. The commonly used drugs such as anti-malarials [16], anti-depressants [18, 22, 32], antibiotics [20, 33], bronchodilators [40], steroids [12, 41], antihypertensives [19, 23, 30, 31, 39], and anti-virals had a gender-specific effect. Examples included studies of steroidal drugs such as Rocuronium and Pancuronium, in which women were 30% more sensitive when compared to men. Women had a higher plasma drug concentration in various types of medication such as valproic acid [17], Desvenlafaxine [18], Carvedilol [19], Encenicline [21], Clomipramine [42], Amiodarone [23], Delavirdine [25, 43], oxcarbazepine and carbamazepine [44], Metoprolol [30], etc. Likewise, women had a lower volume of distribution for Methamphetamine [45], Levofloxacin [33], Albuterol [40], and Ofloxacin [20], and a slower rate of clearance for Verapamil [39], Parampanel [37], and Rosuvastatin [38, 46], etc.
Most of the studies indicated that women tend to have a lower body weight, a higher amount of body fat, lower plasma volume, and a metabolic rate which is higher for cytochrome P450 (CYP) 3A4 substrates, and a lower hepatic activity for the drug efflux transporter P-glycoprotein than men [12, 16, 19, 38, 39, 41, 42, 46]. In contrast to earlier findings, however, we also found that the digestion factor could modify the pharmacological action of the medication. Women are physiologically liable to produce less gastric acid than men because of their slower digestion of foods [19, 42]. Finally, the studies mentioned that the menstrual cycle and steroid hormones are also responsible for modifying a drug’s action [24, 27, 28, 34–36, 47].
Discussion
We obtained 26 studies which provide evidence about the factors which are mainly responsible for altering drugs pharmacokinetics and pharmacodynamics. We identified gender-specific differences for numerous molecular and physiological factors affecting the pharmacokinetics of therapeutic agents, and these pharmacokinetic differences might result in variation of the pharmacological response of men and women. Gender-specific differences in drug distribution might be expected because of the different proportions of muscular and adipose tissue in men and women [48]. The 26 studies overall agreed out that women usually have a lower body weight, shorter organ sizes, and lower plasma volume and blood flow, as well as a higher percentage of body fat. Table 2 shows the basic fundamental difference between men and women that could change the pharmacological action of drugs. However, the systemic exposure and the average concentration of drugs in a steady state depend on its clearance, difference in volume of distribution, and the resulting modification of half-life which are all relevant to the peak which is attained after administrating drugs.
There are several possible explanations for this effect. It is interesting to note that in all the 26 included studies, some female hormones may modify gastric acid secretion, and therefore, gastric PH can lead to slower gastric emptying time in women [49–51]. This could change the significant delay of the onset of an effectiveness of enteric-coated forms, and drugs solubility, as well as dissolution [52]. Table 3 shows the reason for gender-specific differences in drug absorption. However, women usually have a lower organ blood flow which diminishes the blood flow and may thus cause a slower rate and probability lower extent of drug absorption [53]. Higher plasma level is reached in women when compared to men in oral drugs such as ciprofloxacin, oxafloxacin, levofloxacin, gatifloxacin, etc. [54–58], but they also indicate that this difference disappears when the data are normalized by the body weight of an individual.
The most obvious finding to emerge from our review is that the plasma volume, body mass index, average organ blood flow, total body water, and body fat difference between men and women also change the distribution as well as the entire pharmacokinetic process [1, 59–61]. Hydrophilic drugs such as atenolol [62] and ranitidine [63] tend to stay in the blood and the fluid which surrounds the cells [64]. Similarly, Arthur (1994) identified alcohol and ranitidine which revealed a smaller volume of distribution and produced a higher Cmax in women [65]. Other researchers mentioned that due to body fat variation, women have a higher plasma volume of distribution when they intake lipophilic drugs like benzodiazepine [66, 67]. This is because lipophilic drugs have an inclination to be concentrated in fatty tissues. Table 4 lists some drugs whose distribution rate varies between men and women.
Another possible explanation for this is that the drug’s metabolism was the primary focus to explain gender-specific differences in the pharmacokinetics of medicines [49]. For example, the activity of the enzyme pathway in men and women is different. Several enzymatic pathways are reduced in women, whereas, in other cases, the channels are increased in women. Table 5 shows different enzymatic pathways that play a crucial role in drug metabolism in response to gender factors. Tsutsumi et al. [68] described CYP1A which is more prevalent and led to genetic polymorphisms with the extensive metabolizer phenotype. Several studies analyzing metabolite ratios confirmed that men have a higher rate of clearance when caffeine is administered intravenously or orally [69–72]. Furthermore, gender-specific differences in clearance of CYP1A2 substrates were observed in the case of clozapine, olanzapine, and theophylline [49]. Increased levels of estrogen and progesterone alter hepatic enzyme activity, which can increase drug accumulation or decrease elimination of some drugs. Female steroid hormones and prolactin play a role in autoimmunity. However, metabolic changes can also depend on hormone levels that change during the menstrual cycle, with the use of oral contraceptives, throughout pregnancy, or during menopause. Although some researchers believe that the sex hormone plays a dominant role in modulating sex-based differences in pharmacokinetics, such a conclusion result is still controversial. Researchers have failed to show any difference in the case of caffeine [69], paracetamol [73], and ropinirole [74] during the menstrual cycle. Likewise, they did not find any sex-related or menstrual cycle-related differences when treating migraine patients with elitriptan [75].
Therefore, it is important to consider gender-specific differences in pharmacotherapy, because a significant amount of studies mentioned that adverse drugs reaction is 50 to 70% more likely in women [76–80]. The overall incidence of suspected adverse drugs reaction in women was 20.6 per 10,000 patient-months of exposure, whereas in men, it was only 12.9 per 10,000 patient-months of exposure [78]. The most common adverse effect in women is neuropsychiatric, whereas rarer adverse effects are cardiovascular [81], gastrointestinal [82, 83], cutaneous allergic disturbance [83], blood dyscrasias [82], electrolyte disturbances [83], and urinary tract disorder [84]. The Spanish System of Pharmacovigilance reported that 60% of 1609 adverse reactions (OR = 1.67, 95% CI) were due to nonsteroidal anti-inflammatory drugs in women [79]. Moreover, in a review of 93 articles investigating cardiac drugs, 70% of women observed ADRs, even though it is thought there is a male predominance usage of antiarrhythmic drugs [80]. However, anti-infective (60.4%), nervous system agents (21.5%), and musculoskeletal agents (3.7%) reported higher number of ADRs in women [84, 85].
Gender-related dissimilarities in the pharmacokinetics and pharmacodynamics of these drugs have been considered as major determinants for the higher reporting of adverse drugs reactions in women. Likewise, Anderson et al. [52] reported that female patients always had a higher adverse effect of drugs as a consequence of their physiological difference. Several studies showed that female patients have a 1.5- to 1.7-fold greater risk of developing an adverse drug reaction [86, 87], and gender-related drug pharmacokinetics and pharmacodynamics variation play a crucial role in adverse effects [48, 84, 88] (Table 6 ). Much of the research up to now has described adverse effect which occurs due to the type of drugs, administration route, treatment duration, dosage, and bioavailability, but they always ignored gender-specific differences. The rate of adverse effect always varies with patient characteristics which include age, gender, ethnicity, coexisting disorders, and genetic or geographic factors [89–91].
This combination of findings provides some support for the conceptual premise that it is necessary to adjust the dosage or even change medications by gender differentiation. When a patient differently responds to the same amount of dosage, it is recommended that the physician takes into consideration the patient’s sex when they make any decision regarding changing the dose or the medication. Physicians should prescribe medication after considering these differences to minimize the adverse effect and enhance therapeutic effectiveness. The complexity of the female body due to hormonal changes, the menstrual period, the use of birth control pills, and the menopause alters the pharmacological action of drugs due to variation in pharmacokinetics and pharmacodynamics. Nowadays, clinicians are becoming more aware of dissimilarities in the response to treatment of men and women [49, 92], but it is not yet satisfactory. For example, when doctors prescribe medications for pregnant women, particular attention should be paid to drugs treatment, because drugs respond differently during pregnancy [9, 93, 94].
Several questions remain unanswered at present. Since men and women are biologically different, increasing awareness of the possibility of gender on PK/PD variation could influence future clinical trial design. This will create many opportunities to understand the relevance of gender-specific effects, because they certainly do not exist for all drugs, because only 6–7% of those that include a pharmacokinetic gender analysis displaying significant gender differences [52]. It is important to examine whether men and women exhibit different basal expression profiles of drug metabolizing proteins in relevant tissues. Therefore, a human gene expression database is required. This would constitute a large undertaking involving tissues from organ donors and gene expression facilities on a large scale [95]. Moreover, for the issue which is related to non-growth hormone non-drug exposure mechanisms and related to drug metabolizing enzyme or transporter expression, drugs need to be examined on an individual basis.
Our systematic review has several limitations. We did not include race and ethnic factors for PK/PD difference despite racial difference in pharmacokinetics of several drugs having been demonstrated [96]. For example, methylprednisolone clearance was 50% higher in white patients than black patients in a gender- and age-matched study in renal transplants recipients [97]. Black patients were also found to have a different toxicity profile than white patients [98]. Finally, it is not possible to draw conclusions regarding causality through more retrospective observational studies. Therefore, the results of this study should be regarded with caution.
Conclusion
Our study discusses possible reasons for male and female dose differences. As the literature suggests, there are differences how the male and female body deal with drugs because of their differential physiological characteristics. In general, therefore, the current data highlight the importance of involving more females in clinical trials for better results. While the present study is based only on analyzing the published literature, the findings suggest that it may be necessary to differentially adjust the dose for men and women for their safety and efficient treatments. Our study will hopefully serve as a base for future studies and create a better awareness to healthcare providers regarding this issue.
Abbreviations
- BMR:
-
Basal metabolic rates
- CO:
-
Cardiac output
- CYP3A:
-
Cytochrome P450-3A
- GFR:
-
Glomerular filtration rate
- GST:
-
Glutathione-S-transferase isoenzymes
- PGP:
-
p-Glycoprotein
- UGT:
-
Uridine diphosphate glucoronosyl transferase
- ADR:
-
Adverse drug reaction
- CYP1A2:
-
Cytochrome P450-1A2
- GD:
-
Glomerular density
References
Harris RZ, Benet LZ, Schwartz JB (1995) Gender effects in pharmacokinetics and pharmacodynamics. Drugs 50(2):222–239
Rowland M, Tozer T (1989) Assessment of area. In: Clinical pharmacokinetics: concepts and applications (2nd edn). Lea & Febiger, Philadelphia, pp 459–463
Jarugula V, Yeh CM, Howard D, Bush C, Keefe DL, Dole WP (2010) Influence of body weight and gender on the pharmacokinetics, pharmacodynamics, and antihypertensive efficacy of aliskiren. J Clin Pharmacol 50(12):1358–1366
Cheymol G (1993) Clinical pharmacokinetics of drugs in obesity. Clin Pharmacokinet 25(2):103–114
Greenblatt DJ, Friedman H, Burstein ES, Scavone JM, Blyden GT, Ochs HR, Miller LG, Harmatz JS, Shader RI (1987) Trazodone kinetics: effect of age, gender, and obesity. Clin Pharmacol Ther 42(2):193–200
Schwartz AE, Matteo RS, Ornstein E, Young WL, Myers KJ (1991) Pharmacokinetics of sufentanil in obese patients. Anesthesia Analgesia 73 (6):790–793
Lew KH, Ludwig EA, Milad MA, Donovan K, Middleton E Jr, Ferry JJ, Jusko WJ (1993) Gender-based effects on methylprednisolone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 54(4):402
Ensom MH (2000) Gender-based differences and menstrual cycle-related changes in specific diseases: implications for pharmacotherapy. Pharmacother J Hum Pharmacol Drug Ther 20(5):523–539
Koren G (2010) Is it appropriate to study the pharmacokinetics of drugs aimed at pregnant women in men? J Obstet Gynaecol Can 32(7):629–630
Schwartz JB, Capili H, Daugherty J (1994) Aging of women alters S-verapamil pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 55(5):509–517
Anderson GD (2008) Gender differences in pharmacological response. Int Rev Neurobiol 83:1–10
Magee MH, Blum RA, Lates CD, Jusko WJ (2001) Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race. J Clin Pharmacol 41(11):1180–1194
Freedman RR, Sabharwal SC, Desai N (1987) Sex differences in peripheral vascular adrenergic receptors. Circ Res 61(4):581–585
Walker JS, Carmody JJ (1998) Experimental pain in healthy human subjects: gender differences in nociception and in response to ibuprofen. Anesthesia Analgesia 86 (6):1257–1262
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341
Binh VQ, Chinh NT, Thanh NX, Cuong BT, Quang NN, Dai B, Travers T, Edstein MD (2009) Sex affects the steady-state pharmacokinetics of primaquine but not doxycycline in healthy subjects. Am J Trop Med Hyg 81(5):747–753
Ibarra M, Vázquez M, Fagiolino P, Derendorf H (2013) Sex related differences on valproic acid pharmacokinetics after oral single dose. J Pharmacokinet Pharmacodyn 40(4):479–486
Nichols A, Richards L, Behrle J, Posener J, Fruncillo R (2013) Effects of age and sex on the pharmacokinetics, safety, and tolerability of oral desvenlafaxine in healthy adults. J Bioequiv Availab 5:088–094
Abbas M, Khan AM, Riffat S, Tipu MY, Nawaz HA, Usman M (2014) Assessment of sex differences in pharmacokinetics of carvedilol in human. Pak J Pharm Sci 27(5):1265–1269
Zulfiqar-ul-Hassan SR, Naseer R (2008) Gender differences on bioavailabity of ofloxacin. J Ayub Med Coll Abbottabad 20(2):114–117
Barbier AJ, Hilhorst M, Van Vliet A, Snyder P, Palfreyman MG, Gawryl M, Dgetluck N, Massaro M, Tiessen R, Timmerman W (2015) Pharmacodynamics, pharmacokinetics, safety, and tolerability of encenicline, a selective α 7 nicotinic receptor partial agonist, in single ascending-dose and bioavailability studies. Clin Ther 37(2):311–324
Hildebrandt MG, Steyerberg EW, Stage KB, Passchier J, Kragh-Soerensen P (2003) Are gender differences important for the clinical effects of antidepressants? Am J Psychiatry 160(9):1643–1650
Essebag V, Reynolds MR, Hadjis T, Lemery R, Olshansky B, Buxton AE, Josephson ME, Zimetbaum P (2007) Sex differences in the relationship between amiodarone use and the need for permanent pacing in patients with atrial fibrillation. Arch Intern Med 167(15):1648–1653
La Porte C, Burger D, Gyssens I, Sprenger H, Koopmans P (2003) Gender differences in nevirapine pharmacokinetics, fact or fiction. In: Fourth international workshop on clinical pharmacology of HIV therapy, pp 27–29
Smith PF, DiCenzo R, Forrest A, Shelton M, Friedland G, Para M, Pollard R, Fischi M, DiFrancesco R, Morse GD (2005) Population pharmacokinetics of delavirdine and N-delavirdine in HIV-infected individuals. Clin Pharmacokinet 44(1):99–109
Ofotokun I, Chuck SK, Hitti JE (2007) Antiretroviral pharmacokinetic profile: a review of sex differences. Gender Med 4(2):106–119
Pai MP, Schriever CA, Diaz-Linares M, Novak RM, Rodvold KA (2004) Sex-related differences in the pharmacokinetics of once-daily saquinavir soft-gelatin capsules boosted with low-dose ritonavir in patients infected with human immunodeficiency virus type 1. Pharmacother J Hum Pharmacol Drug Ther 24(5):592–599
Fletcher CV, Jiang H, Brundage RC, Acosta EP, Haubrich R, Katzenstein D, Gulick RM (2004) Sex-based differences in saquinavir pharmacology and virologic response in AIDS Clinical Trials Group Study 359. J Infect Dis 189(7):1176–1184
Besi E, Boniface D, Cregg R, Zakrzewska J (2015) Comparison of tolerability and adverse symptoms in oxcarbazepine and carbamazepine in the treatment of trigeminal neuralgia and neuralgiform headaches using the Liverpool Adverse Events Profile (AEP). J Headache Pain 16(1):1
Luzier AB, Killian A, Wilton JH, Wilson MF, Forrest A, Kazierad DJ (1999) Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin Pharmacol Ther 66(6):594–601
Johnson JA, Akers WS, Herring VL, Wolfe MS, Sullivan JM (2000) Gender differences in labetalol kinetics: importance of determining stereoisomer kinetics for racemic drugs. Pharmacother J Hum Pharmacol Drug Ther 20(6):622–628
Härtter S, Wetzel H, Hammes E, Hiemke C (1993) Inhibition of antidepressant demethylation and hydroxylation by fluvoxamine in depressed patients. Psychopharmacology (Berl) 110(3):302–308
Abuelkheir MM (2009) Effect of pharmacokinetic differences between men and women on pharmacodynamic target attainment of levofloxacin against streptococcus pneumoniae. Saudi Pharm J 17(1):29–39
Venuto CS, Mollan K, Ma Q, Daar ES, Sax PE, Fischl M, Collier AC, Smith KY, Tierney C, Morse GD (2014) Sex differences in atazanavir pharmacokinetics and associations with time to clinical events: AIDS Clinical Trials Group Study A5202. J Antimicrob Chemother 69(12):3300–3310
Sargent S, Green S, Para M (1998) Sustained plasma viral burden reductions and CD4 increases in HIV-1 infected patients with RESCRIPTOR (DLV) + RETROVIR (ZDV) + EPIVIR (3TC). In: From: 5th conference on retroviruses and opportunistic infections
Csajka C, Marzolini C, Fattinger K, Décosterd LA, Telenti A, Biollaz J, Buclin T (2004) Population pharmacokinetics of indinavir in patients infected with human immunodeficiency virus. Antimicrob Agents Chemother 48(9):3226–3232
Vazquez B, Yang H, Williams B, Zhou S, Laurenza A (2015) Perampanel efficacy and safety by gender: subanalysis of phase III randomized clinical studies in subjects with partial seizures. Epilepsia 56(7):e90–e94
Macpherson M, Hamrén B, Braamskamp MJ, Kastelein JJ, Lundström T, Martin PD (2016) Population pharmacokinetics of rosuvastatin in pediatric patients with heterozygous familial hypercholesterolemia. Eur J Clin Pharmacol 72(1):19–27
Kang D, Verotta D, Krecic-Shepard ME, Modi NB, Gupta SK, Schwartz JB (2003) Population analyses of sustained-release verapamil in patients: effects of sex, race, and smoking. Clin Pharmacol Ther St Louis 73 (1):31–40
Mohamed MH, Lima JJ, Eberle LV, Self TH, Johnson JA (1999) Effects of gender and race on albuterol pharmacokinetics. Pharmacother J Hum Pharmacol Drug Ther 19(2):157–161
Xue F, Liao X, Liu J, Tong S, Zhang Y, Zhang R, An G, Luo L (1998) Dose-response curve and time-course of effect of vecuronium in male and female patients. Br J Anaesth 80(6):720–724
Hildebrandt MG, Steyerberg EW, Stage KB, Passchier J, Kragh-Soerensen P, Group DUA (2003) Are gender differences important for the clinical effects of antidepressants? Am J Psychiatry 160(9):1643–1650
Friedland GH, Pollard R, Griffith B, Hughes M, Morse G, Bassett R, Freimuth W, Demeter L, Connick E, Nevin T (1999) Efficacy and safety of delavirdine mesylate with zidovudine and didanosine compared with two-drug combinations of these agents in persons with HIV disease with CD4 counts of 100 to 500 cells/mm3 (ACTG 261). JAIDS J Acquir Immune Defic Syndr 21(4):281–292
Besi E, Boniface D, Cregg R, Zakrzewska J (2015) Comparison of tolerability and adverse symptoms in oxcarbazepine and carbamazepine in the treatment of trigeminal neuralgia and neuralgiform headaches using the Liverpool Adverse Events Profile (AEP). J Headache Pain 16(1):1–7
Milesi-Hallé A, Hambuchen MD, McMillan DE, Owens SM (2015) The pharmacokinetics of methamphetamine self-administration in male and female rats. Drug Alcohol Depend 150:164–169
Nazir S, Iqbal Z, Shah Y, Ahmad L, Khan A (2015) Pharmacokinetic study of rosuvastatin in males and females. Eur J Drug Metab Pharmacokinet 40(3):313–318
Ribera E, Lopez RM, Diaz M, Pou L, Ruiz L, Falcó V, Crespo M, Azuaje C, Ruiz I, Ocaña I (2004) Steady-state pharmacokinetics of a double-boosting regimen of saquinavir soft gel plus lopinavir plus minidose ritonavir in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother 48(11):4256–4262
Meibohm B, Beierle I, Derendorf H (2002) How important are gender differences in pharmacokinetics? Clin Pharmacokinet 41(5):329–342
del Carrasco-Portugal MC, Flores-Murrieta FJ (2011) Gender differences in the pharmacokinetics of oral drugs. Pharmacol Pharmacy 2 (01):31
Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF (2004) Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 44:499–523
Coşkun J, Sevinc A, Tevetoğlu I, Alican I, Kurtel H, Yeğen B (1995) Delayed gastric emptying in conscious male rats following chronic estrogen and progesterone treatment. Res Exp Med 195(1):49–54
Anderson GD (2005) Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Women’s Health 14(1):19–29
Schwartz JB (2003) The influence of sex on pharmacokinetics. Clin Pharmacokinet 42(2):107–121
Overholser BR, Kays MB, Forrest A, Sowinski KM (2004) Sex-related differences in the pharmacokinetics of oral ciprofloxacin. J Clin Pharmacol 44(9):1012–1022
Sowinski KM, Abel SR, Clark WR, Mueller BA (1999) Effect of gender on the pharmacokinetics of ofloxacin. Pharmacother J Hum Pharmacol Drug Ther 19(4):442–446
Chien S, Chow A, Natarajan J, Williams R, Wong F, Rogge M, Nayak R (1997) Absence of age and gender effects on the pharmacokinetics of a single 500-milligram oral dose of levofloxacin in healthy subjects. Antimicrob Agents Chemother 41(7):1562–1565
Efthymiopoulos C, Bramer SL, Maroli A (1997) Effect of age and gender on the pharmacokinetics of grepafloxacin. Clin Pharmacokinet 33(1):9–17
Zhang X, Overholser BR, Kays MB, Sowinski KM (2006) Gatifloxacin pharmacokinetics in healthy men and women. J Clin Pharmacol 46(10):1154–1162
Beierle I, Meibohm B, Derendorf H (1999) Gender differences in pharmacokinetics and pharmacodynamics. Int J Clin Pharmacol Ther 37(11):529–547
Pleym H, Spigset O, Kharasch E, Dale O (2003) Gender differences in drug effects: implications for anesthesiologists. Acta Anaesthesiologica Scandinavica 47(3):241–259
Tie H-T, Xia Y-Y, Zeng Y-S, Zhang Y, Dai C-L, Guo JJ, Zhao Y (2014) Risk of childhood overweight or obesity associated with excessive weight gain during pregnancy: a meta-analysis. Arch Gynecol Obstet 289(2):247–257
Custodio JM, Wu C-Y, Benet LZ (2008) Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev 60(6):717–733
Pérez JF, Olguín HJ, Pérez CF, Guillé GP, Pérez AG, Vieyra AC, López AT, Portugal MC, Asseff IL (2003) Effects of gender and phase of the menstrual cycle on the kinetics of ranitidine in healthy volunteers. Chronobiol Int 20(3):499–508
Alomar MJ (2014) Factors affecting the development of adverse drug reactions (review article). Saudi Pharma J 22(2):83–94
Arthur M, Lee A, Wright R (1984) Sex differences in the metabolism of ethanol and acetaldehyde in normal subjects. Clin Sci 67(4):397–401
Kristjansson F, Thorsteinsson S (1990) Disposition of alprazolam in human volunteers. Differences between genders. Acta pharmaceutica nordica 3(4):249–250
Kirkwood C, Moore A, Hayes P, DeVane CL, Pelonero A (1991) Influence of menstrual cycle and gender on alprazolam pharmacokinetics. Clin Pharmacol Ther 50(4):404–409
Tsutsumi K, Kotegawa T, Matsuki S, Tanaka Y, Ishii Y, Kodama Y, Kuranari M, Miyakawa I, Nakano S (2001) The effect of pregnancy on cytochrome P4501A2, xanthine oxidase, and N-acetyltransferase activities in humans. Clin Pharmacol Ther 70(2):121–125
Kalow W, Tang BK (1991) Use of caffeine metabolite ratios to explore CYP1A2 and xanthine oxidase activities. Clin Pharmacol Ther 50(5–1):508–519
Bock KW, Schrenk D, Forster A, Griese E-U, Mörike K, Brockmeier D, Eichelbaum M (1994) The influence of environmental and genetic factors on CYP2D6, CYP1A2 and UDP-glucuronosyltransferases in man using sparteine, caffeine, and paracetamol as probes. Pharm Genom 4(4):209–218
Relling MV, Lin Js, Ayers GD, Evans WE (1992) Racial and gender differences in N-acetyltransferase, xanthine oxidase, and CYP1A2* activities. Clin Pharmacol Ther 52(6):643–658
Ou-Yang DS, Huang SL, Wang W, Xie HG, Xu ZH, Shu Y, Zhou HH (2000) Phenotypic polymorphism and gender-related differences of CYP1A2 activity in a Chinese population. Br J Clin Pharmacol 49(2):145–151
Miners J, Attwood J, Birkett D (1983) Influence of sex and oral contraceptive steroids on paracetamol metabolism. Br J Clin Pharmacol 16(5):503–509
Kaye CM, Nicholls B (2000) Clinical pharmacokinetics of ropinirole. Clin Pharmacokinet 39(4):243–254
Shah AK, LaBoy-Goral M, Scott N, Morse M, Apseloff G (2001) Pharmacokinetics and safety of oral eletriptan during different phases of the menstrual cycle in healthy volunteers. J Clin Pharmacol 41(12):1339–1344
Pouyanne P, Haramburu F, Imbs JL, Bégaud B (2000) Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. Bmj 320(7241):1036
Fattinger K, Roos M, Vergeres P, Holenstein C, Kind B, Masche U, Stocker DN, Braunschweig S, Kullak-Ublick GA, Galeazzi RL (2000) Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 49(2):158–167
Martin RM, Biswas PN, Freemantle SN, Pearce GL, Mann RD (1998) Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol 46(5):505–511
Figueras A, Capella D, Castel J, Laporte J (1994) Spontaneous reporting of adverse drug reactions to non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol 47(4):297–303
Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH (1993) Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. Jama 270(21):2590–2597
Montastruc JL, Lapeyre-Mestre M, Bagheri H, Fooladi A (2002) Gender differences in adverse drug reactions: analysis of spontaneous reports to a Regional Pharmacovigilance Centre in France. Fundam Clin Pharmacol 16 (5):343–346
Klein U, Klein M, Sturm H, Rothenbühler M, Huber R, Stucki P, Gikalov I, Keller M, Hoigne R (1976) The frequency of adverse drug reactions as dependent upon age, sex and duration of hospitalization. International journal of clinical pharmacology biopharmacy 13(3):187–195
Domecq C, Naranjo C, Ruiz I, Busto U (1979) Sex-related variations in the frequency and characteristics of adverse drug reactions. Int J Clin Pharmacol Ther Toxicol 18(8):362–366
Tran C, Knowles SR, Liu BA, Shear NH (1998) Gender differences in adverse drug reactions. J Clin Pharmacol 38(11):1003–1009
Dressler N, Chandra A, Dávila LA, Spineli L, Schippert C, von Versen-Höynck F (2016) BMI and season are associated with vitamin D deficiency in women with impaired fertility: a two-centre analysis. Arch Gynecol Obstet 293(4):907–914
Mattisson D (2008) Sex differences in drug development. Blickpunkt der Mann 6 (1):21–25
Kando JC, Yonkers KA, Cole JO (1995) Gender as a risk factor for adverse events to medications. Drugs 50(1):1–6
Cotreau MM, von Moltke LL, Greenblatt DJ (2005) The influence of age and sex on the clearance of cytochrome P450 3 A substrates. Clin Pharmacokinet 44(1):33–60
Ducharme MP, Slaughter RL, Edwards DJ (1994) Vancomycin pharmacokinetics in a patient population: effect of age, gender, and body weight. Ther Drug Monit 16(5):513–518
Bauer J, Groneberg DA, Brueggmann D (2016) Gender-based workplace assessment in gynecology and obstetrics in Germany: results from the iCEPT Study. Arch Gynecol Obstet 294(2):317–326
Hwang A, Chou L, Islam M, Li Y-C, Syed-Abdul S (2016) Risk factors for ectopic pregnancy in the Taiwanese population: a retrospective observational study. Arch Gynecol Obstet 294(4):779–783
Islam MM, Iqbal U, Walther B, Atique S, Dubey N, Nguyen P-A, Poly T, Masud J, Li Y-C, Shabbir S-A (2016) Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology 47(3–4):181–191
Borgelt LM (2010) Women’s health across the lifespan: a pharmacotherapeutic approach. Can J Hosp Pharm 64(2):153
Hirose A, Terauchi M, Akiyoshi M, Owa Y, Kato K, Kubota T (2016) Low-dose isoflavone aglycone alleviates psychological symptoms of menopause in Japanese women: a randomized, double-blind, placebo-controlled study. Arch Gynecol Obstet 293(3):609–615
Nicolson TJ, Mellor HR, Roberts RR (2010) Gender differences in drug toxicity. Trends Pharmacol Sci 31(3):108–114
Wood AJ, Zhou HH (1991) Ethnic differences in drug disposition and responsiveness. Clin Pharmacokinet 20(5):350–373
Tornatore KM, Reed KA, Venuto RC (1993) Racial differences in the pharmacokinetics of methylprednisolone in black and white renal transplant recipients. Pharmacother J Hum Pharmacol Drug Ther 13(5):481–486
Tornatore KM, Biocevich DM, Reed K, Tousley K, Singh JP, Venuto RC (1995) Methylprednisolone pharmacokinetics, cortisol response, and adverse effects in black and white renal transplant recipients. Transplantation 59(5):729–736
Soldin OP, Mattison DR (2009) Sex differences in pharmacokinetics and pharmacodynamics. ClinPharmacokinet 48(3):143–157
Acknowledgements
This research is sponsored in part by the Ministry of Science and Technology (MOST) under grant MOST 103-2221-E-038-014-, MOST 103-2221-E-038-016-, MOST 104-2221-E-038-013, and MOST 104-3011-E-038 -001; Health and welfare surcharge of tobacco products grant MOHW104-TDU-B-212-124-001, Ministry of Education, Taiwan, under grant TMUTOP103006-6.
Author contributions
Md. Mohaimenul Islam: writing manuscript and data collection. Jakir Hossain Bhuiyan Masud; Usman Iqbal; Suleman Atique, Navneet Kumar Dubey; Phung-Anh Nguyen; Tahmina Nasrin Poly: data collection and arranging manuscript. Bruno Andreas Walther: edit the manuscript. Yu-Chuan (Jack) Li; Shabbir Syed-Abdul: protocol development and manuscript checking.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Since this is a review paper, ethical considerations are not applicable.
Conflict of interest
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Islam, M.M., Iqbal, U., Walther, B.A. et al. Gender-based personalized pharmacotherapy: a systematic review. Arch Gynecol Obstet 295, 1305–1317 (2017). https://doi.org/10.1007/s00404-017-4363-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4363-3