Abstract
Purpose
Prevention of postpartum haemorrhage (PPH) is essential in the pursuit of improved health care for women. Oxytocin, the most commonly used uterotonic agent to prevent PPH, has no established the route of administration. In this study we aimed to compare whether the mode of oxytocin administration, i.e., intravenous and intramuscular administration, has an effect on the potential benefits and side effects.
Materials and methods
A total of 256 women were randomised into two groups: intramuscular group (128) or intravenous group (128).
Results
Estimated blood loss during the third stage of labour was similar between the two groups (p = 0.572). Further there were no statistically significant difference was noted between the two groups in terms of the mean duration of labor, duration of the third stage of labor, manual removal of the placenta, need for instrumental delivery, need for blood transfusion, PPH ≥500 mL, PPH ≥1000 mL, or length of hospital stay.
Conclusion
Using oxytocin by intravenous and intramuscular route has a similar efficacy and adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pregnancy and child birth involve health risk for women even without pre-existing health problems. Maternal mortality and morbidity are the leading contributors of disease burden amongst women. Postpartum hemorrhage (PPH) usually ranks in the top four causes of maternality in developing countries [1]. PPH is defined as blood loss ≥500 mL within 24 hours of birth [2]. The most common underlying cause of PPH is uterine atony [3, 4]. Uterotonic drugs are routinely administered for the active management of the third stage of labor. Oxytocin is regarded as the gold standard uterotonic agent [5] and has been recommended as the first-line prophylactic agent against PPH by many clinical studies [6, 7].
Most oxytocin preparations are administered by injection. However, there exist certain differences between intravenous and intramuscular injections of oxytocin, such as the time taken until the drug starts to work, sudden drop in blood pressure, and an increase in the heart rate. Further, intravenous injection requires sterile conditions, protection from light, and accurate dosing; these factors limit its use in poorly equipped areas [8]. In addition, the intramuscular injection of oxytocin requires lesser time and relatively lesser skills than its intravenous injection.
This paper describes the findings of a randomized trial that assessed the difference between intravenous and intramuscular oxytocin administration in its effectiveness in reducing bleeding after birth. Further, we aimed to examine whether the mode of oxytocin administration, i.e., intravenous and intramuscular administration, has an effect on the potential benefits like decreased uterin bleeding and side effects.
Materials and methods
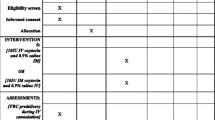
This randomized study was carried out at Bakirkoy Dr. Sadi Konuk Teaching and Research Hospital from February 2014 to March 2015. A total of 256 cases of vaginal deliveries were enroled in this study after obtaining informed consent. The Non-invasive Human Research Ethics Committee and Local Health Ministry Authority approved the study (Approval number 2014-02-02, ClinicalTrials.gov Identifier NCT02080104).
The study inclusion criteria were as follows: women aged 18–45 years with a singleton pregnancy in a cephalic presentation between 37 and 42 weeks gestation and normal blood pressure (<140/90 mmHg) who intended to deliver vaginally. Exclusion criteria were grand multiparity hemoglobin (Hb) <7 g/dL; prolonged first stage of labor induction (oxytocin administration for at least 12 h) [9]; previous Caesarean delivery or uterine surgery; presence of uterine myoma; history of cardiac, hepatic, renal, or neurological disorders; history of coagulopathies and anticoagulation treatment around the time of delivery, history of diabetes or pre-eclampsia; history of postpartum hemorrhage in previous pregnancies or hemorrhage during current pregnancy; and history of placental abruption, macrosomia, or polyhydramnios in the current pregnancy.
Eligible participants were recruited and randomly allocated into two equal groups, i.e., the intravenous and intramuscular oxytocin administration groups, using a random number table. A detailed history was taken from each woman, and general physical examinations were performed. Allocation to groups was not performed until the delivery was imminent. The findings of the initial obstetric evaluation and the follow-up were recorded via partogram. Blood samples were obtained before the delivery to measure hemoglobin (Hb) and hematocrit (Hct) levels. When required, mediolateral episiotomy was performed under local anesthesia. The duration of labor augmentation with oxytocin, if needed, was recorded.
Immediately after the delivery, the amniotic fluid was drained; then, a specially designed blood collection bag was placed beneath the patient and delivery bed to collect and record the blood loss. All participants received 10 IU of oxytocin (Postuitrin-Fort ampule 5 IU, İ.E. Ulagay, Istanbul, Turkey). In both groups, oxytocin was administered after the delivery of the anterior shoulder. In the intravenous group, 10 IU oxytocin was administered via 1000 mL saline solution intravenously at 1 mL/min.
The placenta was removed manually if it was not delivered in 30 min after delivery. If there was excessive vaginal bleeding, the uterus was massaged bimanually for at least 15 seconds [10] and additional uterotonics (20 IU oxytocin in 1000 mL saline solution and intramuscular methylergometrine maleate 0.2 mg) were used. Maternal observations were recorded every 15 min in the first hour and every 30 min in the second hour after delivery. The occurrence of side effects including shivering, pyrexia, nausea/vomiting, and tachycardia was also recorded during the 24 h after the delivery.
In case of perineal tears or episiotomy, a tampon was inserted in the vagina after delivery. The weights of the tampons used were included in the measurement of blood loss. The weights of the gauzes used during episiotomy and perineal repair were not included. Blood-soaked gauzes, gowns, sheets, and tampons were all weighed before and after use. A digital scale (with a 1-g error range) was used for measuring the weights of the gauzes, gowns, sheets, and tampons. Blood loss was estimated using the method described by Gai et al. [11] as follows:
Hb and Hct were re-measured at 24 h postpartum.
The primary outcome measures of the trial were the amounts of blood loss during the delivery and the occurrence of primary PPH. Primary PPH was defined as blood loss of 500 mL or greater within the first 24 hours after delivery of the baby. The secondary outcome measures were as follows: requirement for blood transfusion, total duration of the third stage of labor, incidence of prolonged third stage (i.e., longer than 30 min), the use of additional uterotonics, side effects of oxytocin, and the need for manual removal of the placenta.
Sample size estimation
The mean blood loss in vaginal delivery with standard procedures was approximately 300 ± 205 mL. We hypothesized that a reduction of 25 % in the total amount of blood loss was accepted as clinically significant. At this level of significance, the sample size influence was 0.36, the α error was 0.05, and the β error was 80 %. A sample size of 119 in each group would be required to detect such a difference in blood loss. Assuming a 10 % dropout rate, 128 women were required in each study group.
Statistical analysis
Data analysis for statistical evaluation was performed using the Number Cruncher Statistical System (NCSS) version 2007 (Kaysville, Utah, USA). Data were analyzed using descriptive statistical methods (mean, standard deviation, median, frequency, rate, Minimum, Maximum). For group comparisons of parameters with quantitative data showing normal distribution, Student’s t test was used, while Mann–Whitney U test was used for comparing other parameters not showing normal distribution. For the comparison of qualitative data, Pearson’s Chi square test, Fisher’s exact test, and Yates’ correction continuity (Yates Chi square) test were used. In the follow-up evaluation of the variables within the group, the paired t test was used. A p values less than 0.05 was considered statistically significant.
Results
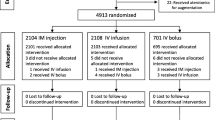
We recruited a total of 256 subjects, out of whom, 128 were randomised to receive oxytocin intramuscularly and 128 were intravenously. Sixty-four of these cases were withdrawn because of not meeting the inclusion criterias. The trial profile is shown in Fig. 1.
The age of the women who participated in this study ranged from 18 to 43 years, and the mean age of the patients was 25.7 years. The parity of the patients ranged from 1 to 6. The gestational age ranged from 37 to 41 weeks as calculated from the first day of the last menstrual cycle. The fetal birth weight ranged from 1905 to 4360 g. Demographic and clinical characteristics were similar between the two treatment groups (Table 1).
Table 2 summarizes the blood pressure and Hb levels in the two groups. The antepartum and postpartum blood pressures in the two groups were not significantly different. Further, there were no statistically significant differences between the two groups in terms of the prepartum Hb (p = 0.349) levels or the postpartum decrease in Hb (p = 0.941; p > 0.05).
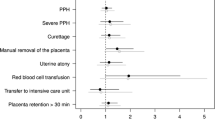
Episiotomy was performed in 46 women (35.9 %) in the intramuscular group and in 53 women (41.4 %) in the intravenous group. One-third/fourth degree perineal laceration was recorded in each group. In the first stage of delivery, 18 and 28 patients received oxytocin in the intramuscular and intravenous groups, respectively. The need for additional uterotonic treatments to prevent PPH was significantly higher in the intravenous group than in the intramuscular group (p = 0.033; p < 0.05).There was no significant difference between the two groups in terms of the mean blood loss from the delivery of the fetus to placental delivery (p = 0.572).
Further, no statistically significant difference was noted between the two groups in terms of the mean duration of labor, duration of the third stage of labor, manual removal of the placenta, need for instrumental delivery, need for blood transfusion, PPH ≥500 mL, PPH ≥1000 mL, or length of hospital stay (Table 3). Similarly, during the first 24 hours postpartum, no statistically significant difference was noted between the two groups for the side effects of oxytocin, which included shivering, nausea and/or vomiting, pyrexia, and tachycardia.
Discussion
PPH is a strong indicator of maternal mortality [12]. A Cochrane review comparing the administration of prophylactic oxytocin with no uterotonics found that both intramuscular and intravenous routes were effective in reducing PPH [13]. In 2012, WHO published a new guideline for the prevention and treatment of PPH. In this guideline, oxytocin (10 IU) was recommended as the first choice of uterotonic drug; however, the route of administration was optional [14]. In the present study, we compared the safety and efficacy of intramuscular and intravenous administration of oxytocin in the prevention of PPH.
The primary outcome in our study was the amount of PPH. Our study showed no significant decrease in hemorrhage when oxytocin was used intramuscularly compared with that after intravenous administration. Further, in our study, no significant difference was noted between the two groups in the 24-hours postpartum decrease in Hb and Hct. This finding is compatible with the findings of Davies et al. [15]. However, in our study, we noted higher additional use of uterotonic drugs in the intravenous group as compared to that in the intramuscular group.
We administered oxytocin before the expulsion of the placenta and cord clamping but immediately after the delivery of the anterior shoulder. It has been reported that the administration of oxytocin before or after the expulsion of the placenta does not have a significant influence on the incidence of PPH and severe PPH, retained placenta, postpartum changes in Hb, the need for blood transfusion, the use of additional uterotonic drugs, or duration of the third stage of labor [16].
The uterine response to the intravenous administration of oxytocin is almost instantaneous as compared with intramuscular administration, taking approximately 5–7 min to work [17]. When administered intravenously, oxytocin leads to an almost immediate action and reaches a plateau concentration after 30 min. In contrast, intramuscular administration results in a slower onset of action but produces a longer lasting clinical effect [18]. However, there are some practical difficulties in administering oxytocin intravenously. Further, intravenous access is not always possible, especially in poorly equipped areas of Turkey. Therefore, in most maternity homes in such areas, oxytocics are administered intramuscularly since intramuscular injection is quicker to administer and requires relatively lesser skills than intravenous injection; thus, it can be administered by midwives and nursing staff.
In our study, the incidence of side effects did not differ statistically between the two groups. Intravenously injected oxytocin is known to induce chest pain, transient profound tachycardia, hypotension, and electrocardiogram changes suggestive of myocardial ischemia [19, 20]. However, there is a paucity of data regarding the side effects of intramuscular oxytocin, probably because there are few side effects of clinical importance. However, the usual side effects of any intramuscular injection, such as pain at injection site and abscess at the injection site when safety procedures are not followed, are to be expected.
In summary, the present study found that the mode of oxytocin administration did not have a significant effect on postpartum blood loss. Multicenter studies with higher sample sizes and meta-analyses are required to establish optimal treatment strategies for the prevention of PPH.
References
Priya GP, Veena P, Chaturvedula L, Subitha L (2015) A randomized controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum hemorrhage. Arch Gynecol Obstet 292:1231–1237. doi:10.1007/s00404-015-3763-5
World Health Organization (2009) WHO guidelines for the management of postpartum haemorrhage and retained placenta
Kaul V, Bagga R, Jain V, Gopalan S (2006) The impact of primary postpartum hemorrhage in ‘Near-Miss’ morbidity and mortality in a tertiary care hospital in North India. Indian J Med Sci 60:233–240
Lewis G (2007) The Confidential Enquiry into Maternal and Child Health (CEMACH) saving mother’s lives: reviewing maternal deaths to make motherhood safer, 2003–2005. The seventh report on Confidential Enquiry into Maternal Deaths in the United Kingdom. CEMACH, London
Wedisinghe L, Macleod M, Murphy DJ (2008) Use of oxytocin to prevent haemorrhage at caesarean section—a survey of practice in the United Kingdom. Eur J Obstet Gynecol Reprod Biol 137:27–30
Bouwmeester FW, Bolte AC, van Geijn HP (2005) Pharmacological and surgical therapy for primary postpartum hemorrhage. Curr Pharm Des 11:759–773
Davies GA, Tessier JL, Woodman MC, Lipson A, Hahn PM (2005) Maternal hemodynamics after oxytocin bolus compared with infusion in the third stage of labor: a randomized controlled trial. Obstet Gynecol 105:294–299
Miller S, Lester F, Hensleigh P (2004) Prevention and treatment of postpartum hemorrhage: new advances for low-resource settings. J Midwifery Womens Health 49:283–292
Rouse DJ, Owen J, Hauth JC (2000) Criteria for failed labor induction: prospective evaluation of a standardized protocol. Obstet Gynecol 96:671–677
Chong YS, Su LL, Arulkumaran S (2004) Current strategies for the preventionof postpartum hemorrhage in the third stage of labor. Curr Opin Obstet Gynecol 16:143–150
Ying Gai Ming, Wu Lian-fang Su, Qi-feng Karin Tatsumoto (2004) Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi-center, randomized trial. Eur J Obstet Gynecol Reprod Biol 112:154–157
Carroli G, Cuesta C, Abalos E, Gulmezoglu AM (2008) Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol 22:999–1012
Cotter AM, Ness A, Tolosa JE (2001) Prophylactic oxytocin for the third stage of labour. Cochrane Database Syst Rev (4)
Westhoff G, Cotter AM, Tolosa JE (2013) Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database Syst Rev 10:CD001808. doi:10.1002/14651858.CD001808.pub2
Davies GA, Tessier JL, Woodman MC, Lipson A, Hahn PM (2011) Maternal hemodynamics after oxytocin bolus compared with infusion in the third stage of labor: a randomized controlled trial. Obstet Gynecol 105:294–299
Soltani H, Hutchon DR, Poulose TA (2006) Timing of prophylactic uterotonics for the third stage of labour after vaginal birth. Cochrane Database Syst Rev (4)
Jackson KW Jr, Allbert JR, Schemmer GK, Elliot M, Humphrey A, Taylor J (2001) A randomized controlled trial comparing oxytocin administration before and after placental delivery in the prevention of postpartum hemorrhage. Am J Obstet Gynecol 185:873–877
Breathnach F, Geary M (2006) Standard medical therapy. In: Blynch C, Keith LG, Lalonde AB, Karoshi M (eds) A textbook of postpartum haemorrhage. Sapiens Publishing, Duncow, pp 256–262
Charbit B, Funck-Brentano C, Samain E, Jannier-Guillou V, Albaladejo P, Marty J (2007) QT interval prolongation after oxytocin bolus during surgical induced abortion. Clin Pharmacol Ther 76:359–364
Svanström MC, Biber B, Hanes M, Johansson G, Näslund U, Bålfors EM (2008) Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. Br J Anaesth 100:683–689
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dagdeviren, H., Cengiz, H., Heydarova, U. et al. Intramuscular versus intravenous prophylactic oxytocin for postpartum hemorrhage after vaginal delivery: a randomized controlled study. Arch Gynecol Obstet 294, 911–916 (2016). https://doi.org/10.1007/s00404-016-4060-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4060-7