Abstract
Purpose
Glucagon-like peptides receptor agonists are currently approved as anti-obesity agents, yet the experience with their use in polycystic ovarian syndromes (PCOS)-related obesity and insulin resistance is still limited.
Methods
We examined the effects of liraglutide on obesity, insulin resistance, and androgen levels in PCOS through a meta-analysis.
Results
Seven RCTs where women with PCOS were treated with liraglutide were identified. The variables that were examined before and after a 90-day treatment included waist circumference, body mass index (BMI), fasting insulin concentrations, insulin resistance using homeostatic model (HOMA-IR), serum testosterone, and sex hormone-binding globulin (SHBG). The analysis included 178 women. Only 172 patients had post-treatment measurements. While BMI significantly dropped by −1.65 (0.72–2.58) Kg/m2 after 3 months treatment with liraglutide, waist circumference did not change significantly. Similarly, fasting insulin levels, insulin sensitivity, and SHBG did not change significantly. However, serum testosterone decreased by 0.29 nmol/L in 88 women (P = 0.0003).
Conclusion
In a limited number of the women with PCOS, BMI and serum testosterone are only variables that significantly decrease after 3 months of treatment with GLP-1 receptor agonists. Larger sample size studies with longer durations of treatment may be required to examine potential benefits of these medications in improving insulin sensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
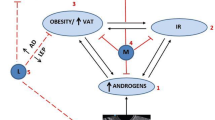
Polycystic ovary syndrome (PCOS) is a common disorder with prevalence as high as 15–20 % of women of reproductive age [1]. The clinical picture commonly includes obesity, hirsutism, oligomenorrhea, and subfertility [2–4]. Obesity is reported in 30–75 % of PCOS women and approximately 50–80 % of them demonstrate varying degree of insulin resistance (IR) [5]. The development of hyperinsulinemia and IR has been thoroughly examined in PCOS. It is generally accepted that in patients with PCOS, the impaired sensitivity to insulin is either related to obesity (mediated through serum concentrations of adiponectin) or it is merely due to androgenic hypersecretion [6, 7]. Patients with PCOS and IR have significantly higher diastolic blood pressure compared to PCOS alone [8]. Based on this current understanding of PCOS, it is important that the patient and medical provider approach management not only toward improving the often troublesome hirsutism and infertility but also toward the long-term risks associated with IR.
Glucagon-like peptide-1 receptors (GLP-1 R) is a gut hormone secreted in response to ingestion of carbohydrates, lipids, and mixed meals from the L-cells located in the distal jejunum, ileum, and colon/rectum [9, 10]. There are currently two GLP-1R agonists available (exenatide and liraglutide), with several more currently being developed. Liraglutide is a GLP-1R analog that reduces blood glucose levels, increases insulin secretion, inhibits glucagon secretion, increases pancreatic β cell growth, suppresses appetite and energy intake [11], delays gastric emptying, and improves insulin sensitivity through mechanisms that are not completely understood [12]. GLP-1R agonists have been used for the treatment of patients with type-2 diabetes mellitus for past 5 years. Liraglutide reduces plasma glucose levels in both fasting and post prandial state, as injected once daily has been shown to improve glycemic control in individuals with type-2 diabetes (up to a 1.5 % decrease in glycated hemoglobin). Additional advantages of GLP-1R agonists include their effect on components of metabolic syndrome other than insulin resistance, such as body weight, blood pressure, and lipid profile [13]. The minimal risk of hypoglycemia makes liraglutide an attractive option for management of insulin resistance in patients with metabolic syndrome.
GLP-1R agonist has recently been introduced as a therapeutic alternative for management of patients with PCOS. There are well-designed meta-analyses based on abundance of evidence that have examined the effects of GLP-1R agonists on body weight, cardiovascular risk and glycemic control in type-2 diabetes mellitus [14–17]. Although there are few clinical trials and observational studies which examined the effects of GLP-1R agonists on IR observed in PCOS, a systematic review of such a treatment is long overdue. The main objective of the present meta-analysis is to assess the effects of GLP-1R agonists on patients with PCOS, separating placebo-controlled trials from comparisons with other active drugs. Insulin resistance was the primary outcome variable in the current meta-analysis and our primary hypothesis was that GLP-1R agonist, liraglutide, while improving insulin sensitivity; it reduced body weight in women with PCOS.
Methods
Information sources
The electronic databases MEDLINE (from 1966 to March 2013) and EMBASE (from 1980 to March 2013), as well as the Cochrane Library were searched for the MESH terms “Glucagon like peptide-1 receptor agonist,” “Liraglutide,” “Insulin Resistance,” and “Metabolic Syndrome.” Only case–control and randomized clinical trials on humans up to May 2015 were collected. We further narrowed the search by using the filters “polycystic ovarian syndrome.” Furthermore, we reviewed reference lists of original and reviewed articles to search for more studies.
Study selection
Only those studies that were published as full-length articles were considered. No language restriction was applied. When confronted with different time treatment for evaluation of GLP-1R agonists, 3 months of treatment was considered for final analysis rather than 6 months duration of treatment. Narrowing the search for “polycystic ovarian syndrome” and or “metabolic syndrome” results in articles. After careful examination of titles and abstracts, a total of 310 citations were identified from databases. Additional 16 articles were identified through search from other databases and from the references of articles. 297 records were excluded for their irrelevant design and patient population. The full-text contents were examined from 28 publications. Three investigative teams published a total of ten manuscripts, with a direct relevance to the subject of this meta-analysis. One study mainly examined the genetic variability of GLP-1R in women with PCOS and did not examine the endocrine and/or components of metabolic syndrome in this population. From nine studies, three studies contained duplicate material and therefore were excluded. Finally, six case-controlled studies were identified for analysis.
Data items
From the components of metabolic syndrome, waist circumference, body mass index (BMI), systolic blood pressure, total serum testosterone, sex hormone-binding globulin (SHBG) levels in the plasma, fasting insulin concentration, and homeostatic model assessment for insulin resistance (HOMA-IR) scores were used as continuous outcome variables. The effect size was examined for these variables comparing the post-treatment values to their pre-treatment levels. The presence or absence of insulin resistance was uniformly identified by all studies as HOMA scores >2.60. This variable was the only dichotomous variable for which the results were presented as the overall odds ratio.
Eligibility criteria
For inclusion, studies had to fulfill the following criteria: (a) original article with a sample size of five or more participants; (b) adult population; (c) reporting the values of the outcome variables both at baseline pretreatment and after 3 months of treatment with a GLP-1 agonist; (d) evaluation of metabolic syndrome by Rotterdam criteria and (e) evaluation of insulin resistance by HOMA-IR scoring system. We excluded studies where the evaluation was completed on patients with diagnosis other than polycystic ovarian syndrome. We also excluded the results of the studies with combined treatment strategy (metformin + liraglutide). We also excluded studies in patients having pre-existing overt diabetes mellitus. If multiple published reports from the same study were available, we included only the one with the most detailed information for both exposure and outcome.
Data extraction
Articles were identified in a staged process whereby titles were initially screened for potential eligibility. Abstracts and full texts of potentially eligible articles were assessed by two reviewers (MN and NDN) independently and any disagreements were resolved by consensus. Data included the first author’s surname, year of publication, country of origin of the population studied, study design, number of participants, and duration used for both exenatide and liraglutide treatment. Whenever needed, we obtained additional information about a specific study by directly contacting the primary author.
Synthesis of results
The Cochrane collaborative review for systemic analysis (RevMan software 5.3) was used to calculate the risk ratio for the incidence of insulin resistance for each study. The effect size was calculated with the overall significance of Z values for all continuous variables. I 2 was used to assess statistical heterogeneity with a value below 30 % standing for low heterogeneity, a value between 30 and 60 % standing for moderate heterogeneity and a value above 60 % standing for high heterogeneity. Fixed effect model was used in all analyses. Risk bias was assessed according to Cochrane review. A P value of 0.05 or less was significant.
Results
Study selection
Seven studies were examined in this meta-analysis. The results from one study duplicated the older study by the same group of investigators [18], and therefore the older study was excluded from analysis. The quantitative outcome variables that were tested before and after 3 months treatment with glucagon-like peptide analogs (liraglutide in six studies and exenatide in one study) included weight, body mass index (BMI), waist circumference, systolic blood pressure, fasting insulin level, and HOMA score. Furthermore, insulin resistance was measured as a dichotomous variable if HOMA scores were greater than 2.60.
Synthesis of results
In one study [19], exenatide was used 10 µg twice a day. In the remaining five studies, liraglutide was used. The starting dose was 0.6 mg/day for 1 week and then increased to 1.2 mg/day for the rest of the study period. In one study [18], researchers increased the dose to 1.8 mg/dL after the second week. All studies included patients older than 18 years. Four of them limited the study to premenopausal women with two others having maximum of 40 and 45 years old as their inclusion criteria. The mean age of women included in this study is 32.8 ± 7.0 years. From anthropometric data, BMI and waist circumference were measured within these studies. BMI was tested in 6 studies [18–23] in which a total of 178 women who were diagnosed with PCOS were treated with liraglutide for a period of 3 months. Six patients dropped of the study and post-treatment measurements were missing. BMI decreased by 1.65 (0.72–2.58) Kg/m2 in 172 patients after 3 months (Fig. 1a). The overall effect of liraglutide on BMI was statistically significant (P = 0.0005) and the comparison had negligible level of heterogeneity (I 2 = 0.0 %). Waist circumference was examined only in four studies (Fig. 1b) [18, 22, 23]. Eighty women with PCOS were examined for this variable at the baseline but only 74 women were available for measurement after completion of the treatment. Waist circumference decreased to 117 cm from its pre-treatment value of 120 cm (95 % confidence interval was −7.10 to 0.97 cm, P = 0.142). This comparison also carried a negligible level of heterogeneity (I 2 = 0.0 %).
Systolic blood pressures were also recorded only in three studies [18, 22, 23]. In 44 patients, the systolic blood pressure modestly decreased 2.5 mmHg (95 % confidence interval range was −8.30 to 3.25 mmHg; P = 0.389) following 3 months treatment with liraglutide. This insignificant change in systolic blood pressure had no heterogeneity (I 2 = 0.0 %).
Fasting serum insulin levels were reported in five studies (Fig. 2a) [19, 22–25]. Among 94 women with PCOS, fasting serum insulin levels were similar before and after 3-month treatment with GLP-1 analogs (95 % confidence interval range was −3.81 to 1.10 mU/L; P = 0.283). HOMA scores were calculated for all 94 women prior to the treatment and in 88 women who completed the treatment plan. There was a trend in decreasing HOMA scores following GLP-1 treatment (Fig. 2b). HOMA scores were 5.73 ± 2.56 prior to the treatment, which reduced by 0.84 following 3-month treatment (95 % confidence interval of −1.79 to 0.12, P = 0.091). The comparison of HOMA scores carried a significant level of heterogeneity (I 2 = 63 %; P = 0.03), while the comparison for fasting insulin levels was completely homogenous.
Insulin resistance as it was determined by HOMA scores greater than 2.60 was reported in 43 out 80 women with PCOS (53.8 %) reported by four studies [22–25]. Following 3-month treatment with GLP-1 analogs, still 33 of 74 women (44.6 %) remained insulin resistant (P = 0.26). The overall odd ratio for insulin resistance calculated by HOMA scores >2.60 was 0.69 (95 % confidence interval ranging from 0.37–1.31). Heterogeneity for this comparison was also zero.
Total testosterone was reported in five of these studies (Fig. 3a) [19, 22–25]. One study reported the testosterone values in ng/dl, which were converted to nmol/L by multiplying to 0.0347 [25]. Following liraglutide treatments, total testosterone decreased from 1.89 nmol/L in 97 patients to 1.60 nmol/L in 88 patients (95 % confidence interval ranging from −0.44 to −0.13; P = 0.0003). However, there was no significant difference in concentrations of sex hormone-binding globulin (SHBG) after treatment with GLP-1 analogs (Fig. 1b). SHBG was 26.7 ± 18.2 nmol/L in 97 women with PCOS, which increased to 28.3 ± 15.8 nmol/L after treatment with GLP-1 analogs (95 % confidence interval ranging from −1.13 to 4.41; P = 0.253). Heterogeneity was negligible for both total testosterone and SHBG concentrations (I 2 = 0.0 %). LH and FSH levels were measured in two studies [21, 22]. Following liraglutide treatment, LH increased insignificantly by 1.89 I.U./L in 50 patients (95 % confidence interval ranging from −1.53 to 5.31 I.U./L; P = 0.17). Following GLP-1 agonist treatment, FSH changed by 0.48 IU/L in 50 patients (95 % confidence interval ranging from −0.32 to 1.28; P = 0.81). DHEA-S levels were reported by three studies in 64 women [19, 21, 22]. One study reported the DHEAS levels in µmol/L, which were converted to µg/dL. Following GLP-1 agonist treatment, DHEAS had a minor increase by 0.26 µg/dL in 64 patients (95 % confidence interval ranging from −1.36 to 1.36 µg/dL; P = 0.82).
One study reports no significant changes in Ferriman-Gallwey scores for hirsutism and menstrual cycles per year post-treatment with liraglutide [19].
Discussion
Summary of evidence
The role of obesity, IR, and hyperinsulinemia in the development of PCOS has been thoroughly explored, and it is generally accepted to play an important role in the molecular mechanisms implicated in the androgenic hypersecretion typical of this pathology [7]. The few available trials designed with weight loss as the principal endpoint and enrolling non-diabetic patients with obesity have shown that GLP-1 receptor agonists have a potential use as drugs for the treatment of obesity [26, 27]. As much as 5–10 % weight loss improves clinical (reproductive and metabolic) outcomes in PCOS women [28, 29]. Metabolic syndrome is commonly seen in women with PCOS. This syndrome includes abdominal obesity, systolic hypertension, hyperlipidemia in the form increased serum triglyceride concentrations, and insulin resistance.
In a systematic review and meta-analysis of effects of GLP-1 agonists, mean weight loss of 3.2 and 2.8 kg was reported in obese patients without and with diabetes, respectively [16]. One trial in non-diabetic obese patients reported a BMI reduction of 0.600 kg/m2 compared to placebo at 6 months follow-up [27]. In a meta-analysis of effects of GLP-1 receptor agonists on weight reduction in obese diabetic patients, a 1 kg/m2 reduction in BMI was reported [14]. These numbers are comparable to 1.65 kg/m2 reduction in BMI that we observed in this review.
Limitations
The present meta-analysis included data from case-controlled trials assessing clinically relevant doses of GLP-1R agonists given for at least 12 weeks. All of the analyzed studies were on women with PCOS. The outcome variables assessed in this meta-analysis included anthropometric data such as body mass index and waist circumference, fasting serum insulin levels, and insulin resistance as assessed by homeostatic model assessment of insulin resistance as the components of metabolic syndrome, as well as androgenic status of the patients like serum total testosterone levels and sex hormone-binding globulin (SHBG) concentrations. The six selected studies obtained the baseline measurement from a total of 178 women; however, they reported post-treatment variables from 172 women. Six PCOS patients were lost to follow up in one of the studies [25, 30]. Although all the factors were not examined in these studies uniformly, there was acceptable homogeneity in comparing aforementioned variables in PCOS patients before and after 12-week treatment with GLP-1R agonists. Majority of the studies compared GLP-1R analogs to metformin in changing anthropometric, endocrine, and insulin resistance. Additional studies compared the effect of treatment in obese women with PCOS to obese women without this syndrome.
Women with PCOS demonstrate varying degree of resistance to insulin, and frequently present with elevated levels of serum insulin [5, 31, 32]. Insulin resistance in PCOS is due to multiple interacting mechanisms. Obesity and high levels of androgens both may contribute to the observed resistance to insulin [33, 34]. Decreased density of insulin receptors relative to the enlarged adipose tissue mass has been shown to contribute to insulin resistance in obese patients [35]. Due to the lack of negative feedback, overstimulation of beta islet cells results in insulin overproduction. The effects of testosterone on insulin sensitivity are even less clear and controversial. While there is evidence that men with lower testosterone levels are more prone to develop insulin resistance [36], testosterone by itself has been described to decrease insulin sensitivity by inducing decreased capillary density in peripheral muscle tissue [34]. Conversely, elevated levels of serum insulin shift the progesterone cycle within the ovaries to produce excessive amounts of testosterone in non-diabetic adults [37, 38]. Similar effects have been described in men involving Leydig cells within the testes [36, 39].
Conclusions
We found that HOMA-IR had a trend in decreasing following 3 months of GLP-1 treatment but this difference was not significant. The results of our analyses indicate that the treatment with GLP-1R agonists help reduce body weight in PCOS patients who are overweight or obese. Reduction in BMI was not associated with any decrease in waist circumference, indicating that GLP-1R agonist rather affected the overall obesity than the abdominal obesity. This finding is consistent with similar studies reported in patients with no metabolic syndrome or polycystic ovaries. There are few studies reporting treatment success with GLP-1R agonists and a reduction in the homeostasis model of insulin resistance [12, 40]. GLP-1R analogs improved insulin sensitivity and showed insignificant decrease in fasting insulin level in women with PCOS. This finding was interesting since GLP-1R agonists are shown to increase endogenous insulin secretions both in diabetics and non-diabetics. The observed dichotomy may be related to the improved insulin sensitivity, which in turn lowers the fasting levels of insulin in this patient population.
References
Sirmans SM, Pate KA (2013) Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 6:1–13. doi:10.2147/CLEP.S37559
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO (2004) The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89(6):2745–2749. doi:10.1210/jc.2003-032046
Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS (1995) Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 10(8):2107–2111
Glueck CJ, Dharashivkar S, Wang P, Zhu B, Gartside PS, Tracy T, Sieve L (2005) Obesity and extreme obesity, manifest by ages 20–24 years, continuing through 32–41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol 122(2):206–212. doi:10.1016/j.ejogrb.2005.03.010
Legro RS, Castracane VD, Kauffman RP (2004) Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv 59(2):141–154. doi:10.1097/01.OGX.0000109523.25076.E2
Niafar M, Nader ND (2015) Adiponectin as serum biomarker of insulin resistance in patients with polycystic ovarian syndrome. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. doi:10.3109/09513590.2015.1008445
Diamanti-Kandarakis E, Dunaif A (2012) Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33(6):981–1030. doi:10.1210/er.2011-1034
da Silva AM, de Andrade AC, Dias BH, da Silva Medeiros MA, Rao VS, das Chagas Medeiros F (2014) Elevated diastolic blood pressure in insulin-resistant polycystic ovarian syndrome patients. Arch Gynecol Obstet 289(1):119–122. doi:10.1007/s00404-013-2953-2
Holst JJ (1994) Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology 107(6):1848–1855
Kreymann B, Williams G, Ghatei MA, Bloom SR (1987) Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 2(8571):1300–1304
Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O (2007) Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care 30(8):2032–2033. doi:10.2337/dc07-0310
Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B, Group L-S (2009) Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373(9662):473–481. doi:10.1016/S0140-6736(08)61246-5
Derosa G, Maffioli P (2012) GLP-1 agonists exenatide and liraglutide: a review about their safety and efficacy. Curr Clin Pharmacol 7(3):214–228
Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E (2012) Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res 2012:672658. doi:10.1155/2012/672658
Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E (2014) Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 16(1):38–47. doi:10.1111/dom.12175
Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL (2012) Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344:d7771. doi:10.1136/bmj.d7771
Du Q, Wang YJ, Yang S, Zhao YY, Han P (2014) Liraglutide for the treatment of type 2 diabetes mellitus: a meta-analysis of randomized placebo-controlled trials. Adv Ther 31(11):1182–1195. doi:10.1007/s12325-014-0164-2
Kahal H, Aburima A, Ungvari T, Rigby AS, Coady AM, Vince RV, Ajjan RA, Kilpatrick ES, Naseem KM, Atkin SL (2015) The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocr Disord 15:14. doi:10.1186/s12902-015-0005-6
Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R (2008) Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 93(7):2670–2678. doi:10.1210/jc.2008-0115
Rasmussen CB, Lindenberg S (2014) The effect of liraglutide on weight loss in women with polycystic ovary syndrome: an observational study. Front Endocrinol (Lausanne) 5:140. doi:10.3389/fendo.2014.00140
Jensterle M, Kocjan T, Kravos NA, Pfeifer M, Janez A (2015) Short-term intervention with liraglutide improved eating behavior in obese women with polycystic ovary syndrome. Endocr Res 40(3):133–138. doi:10.3109/07435800.2014.966385
Jensterle M, Kravos NA, Pfeifer M, Kocjan T, Janez A (2015) A 12-week treatment with the long-acting glucagon-like peptide 1 receptor agonist liraglutide leads to significant weight loss in a subset of obese women with newly diagnosed polycystic ovary syndrome. Hormones (Athens) 14(1):81–90
Jensterle Sever M, Kocjan T, Pfeifer M, Kravos NA, Janez A (2014) Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J Endocrinol 170(3):451–459. doi:10.1530/EJE-13-0797
Jensterle M, Kocjan T, Kravos NA, Pfeifer M, Janez A (2014) Short-term intervention with liraglutide improved eating behavior in obese women with polycystic ovary syndrome. Endocr Res. doi:10.3109/07435800.2014.966385
Kahal H, Aburima A, Ungvari T, Rigby AS, Coady AM, Vince RV, Ajjan RA, Kilpatrick ES, Naseem KM, Atkin SL (2015) The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocrine Disord 15(1):14. doi:10.1186/s12902-015-0005-6
Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Hakim A, Madsen J, Rasmussen MF, Lean ME, Group NNS (2009) Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374(9701):1606–1616. doi:10.1016/S0140-6736(09)61375-1
Rosenstock J, Klaff LJ, Schwartz S, Northrup J, Holcombe JH, Wilhelm K, Trautmann M (2010) Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care 33(6):1173–1175. doi:10.2337/dc09-1203
Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ (1998) Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod 13(6):1502–1505
Huber-Buchholz MM, Carey DG, Norman RJ (1999) Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab 84(4):1470–1474. doi:10.1210/jcem.84.4.5596
Kahal H, Abouda G, Rigby AS, Coady AM, Kilpatrick ES, Atkin SL (2014) Glucagon-like peptide-1 analogue, liraglutide, improves liver fibrosis markers in obese women with polycystic ovary syndrome and nonalcoholic fatty liver disease. Clin Endocrinol 81(4):523–528. doi:10.1111/cen.12369
Diamanti-Kandarakis E (2006) Insulin resistance in PCOS. Endocrine 30(1):13–17. doi:10.1385/ENDO:30:1:13
Dunaif A (1997) Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18(6):774–800. doi:10.1210/edrv.18.6.0318
Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R (2002) Obesity and the polycystic ovary syndrome. Int J Obesity Relat Metab Disord J Int Assoc Study Obesity 26(7):883–896. doi:10.1038/sj.ijo.0801994
Rincon J, Holmang A, Wahlstrom EO, Lonnroth P, Bjorntorp P, Zierath JR, Wallberg-Henriksson H (1996) Mechanisms behind insulin resistance in rat skeletal muscle after oophorectomy and additional testosterone treatment. Diabetes 45(5):615–621
Reaven GM (1995) Pathophysiology of insulin resistance in human disease. Physiol Rev 75(3):473–486
Pitteloud N, Hardin M, Dwyer AA, Valassi E, Yialamas M, Elahi D, Hayes FJ (2005) Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab 90(5):2636–2641. doi:10.1210/jc.2004-2190
Nestler JE (1997) Insulin regulation of human ovarian androgens. Hum Reprod 12(Suppl 1):53–62
Poretsky L, Bhargava G, Saketos M, Dunaif A (1990) Regulation of human ovarian insulin receptors in vivo. Metab Clin Exp 39(2):161–166
Pasquali R, Casimirri F, De Iasio R, Mesini P, Boschi S, Chierici R, Flamia R, Biscotti M, Vicennati V (1995) Insulin regulates testosterone and sex hormone-binding globulin concentrations in adult normal weight and obese men. J Clin Endocrinol Metab 80(2):654–658. doi:10.1210/jcem.80.2.7852532
Yang M, Liu R, Li S, Luo Y, Zhang Y, Zhang L, Liu D, Wang Y, Xiong Z, Boden G, Chen S, Li L, Yang G (2013) Zinc-alpha2-glycoprotein is associated with insulin resistance in humans and is regulated by hyperglycemia, hyperinsulinemia, or liraglutide administration: cross-sectional and interventional studies in normal subjects, insulin-resistant subjects, and subjects with newly diagnosed diabetes. Diabetes Care 36(5):1074–1082. doi:10.2337/dc12-0940
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Niafar, M., Pourafkari, L., Porhomayon, J. et al. A systematic review of GLP-1 agonists on the metabolic syndrome in women with polycystic ovaries. Arch Gynecol Obstet 293, 509–515 (2016). https://doi.org/10.1007/s00404-015-3976-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3976-7