Abstract
Purpose
The physiological changes during pregnancy can significantly alter antiepileptic drug (AED)’s absorption, distribution, metabolism and elimination, thus influencing their plasma concentration. Considering that the risks of using old and new AEDs during pregnancy are still debated, our aim is to review the available evidence on this topic.
Methods
Narrative overview, synthesizing the findings of literature retrieved from searches of computerized databases.
Results
The old AEDs generation (benzodiazepines, phenytoin, carbamazepine, phenobarbital and valproic acid) is teratogenic: minor congenital malformations, such as facial dysmorphism and other anomalies, occur in 6–20 % of infants exposed to AEDs in utero; this value is two times greater than the value reported in the general population. Major congenital malformations (MCM) such as cleft lip and cleft palate, heart defects (atrial septal defect, Fallot’s tetralogy, ventricular septal defect, aortic coarctation, patent ductus arteriosus, and pulmonary stenosis) and urogenital anomalies were estimated to be 4–6 % of infants born from mothers treated with AEDs, compared to 2–3 % of the general population.
Conclusion
It is essential to inform women treated with AED that planning pregnancy is necessary, when possible. The problems related to antiepileptic therapy and the possibilities of prenatal diagnosis should be accurately discussed with the patient, when possible before pregnancy: individual circumstances, desire to have children, severity of epilepsy, risks of seizures, family history of congenital malformations and all other potential risk factors must be considered, involving the patient in shared clinical decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a condition characterized by a change of primary electrical activity in the brain, which causes recurrent crisis: it can be focal when originating within the networks limited to one hemisphere or generalized when it involves both of them. It can affect cortical and subcortical structures even without involving the entire cortex; the seizure is a transient event that can occur with typical absences, generalized tonic–clonic and myoclonic crisis, alone or in various combinations; the epileptic syndrome occurs in the absence of structural brain injury, suggesting a genetic aetiology [1]. 65 million people worldwide are affected by epilepsy and the prevalence is estimated to be approximately 700 out of 100.000 people [2, 3]. The prevalence is 15.4 % in sub-Saharan Africa, 5.4 % in Latin America, 12.4 in Europe and 5–10 % in North America [4]. According to the International league against epilepsy (ILAE), epilepsy could be considered as a brain disorder characterized by a persistent predisposition to develop seizures [5]. In clinical practice, this definition is usually applied when there are two unprovoked seizures, separated by a time interval >24 h, and a third seizure in the span of 10 years [5, 6]. After the first occurrence of unprovoked seizures, the recurrence risk is 40–52 % (60–90 % at 4 years) [7, 8]. Considering that the risks of using old and new antiepileptic drugs (AEDs) during pregnancy are still debated, our aim is to review the available evidence on this topic. In this regard, we performed a narrative overview, synthesizing the findings of literature retrieved from searches of computerized databases.
Maternal physiological changes during pregnancy
Maternal changes occurring during pregnancy and involving all organs and systems, physiologically revert after childbirth. The heart increases its output from 40 to 50 % and this is accompanied by a consequent increase in the heart rate and a proportional increase in the stroke volume [9, 10]. Blood and plasma’s volume increase causing a dilution of the haemoglobin concentration, thus the increased red blood cells require an additional contribution of iron [11, 12]. Changes occurring in the urinary system are: increase of the glomerular filtration rate, increase in renal function with consequent nitrogen’s decrease and lower creatinine values [13]. The respiratory system is in part influenced by progesterone and partially offset by the volume of the uterus; concomitant increases in respiratory rate, pH and O2 consumption, and minimal hyperemia and edema of the respiratory tract also occur in some cases [14, 15]. The compression exerted by the uterus on the gastrointestinal and hepatobiliary systems, on the rectum and on the last part of the colon may lead to constipation and gastroesophageal reflux, increasing abdominal pressure [16–18]. Alkaline phosphatase’s level increases during the third quarter of pregnancy [19, 20]. Pregnancy also affects the function of many endocrine glands, in part because the placenta produces hormones and in part because a high percentage of this hormone circulates in blood bound to transporter proteins. In fact, this binding percentage is increased during pregnancy [21]: furthermore, thyroid’s function is increased, as well as the adrenal glands’ one. There are also increased levels of glucocorticoids, estrogens and progesterone, which alter glucose metabolism and insulin requirement [22, 23].
Obstetric management of women with epilepsy
The incidence of epilepsy in the obstetric population undergoing AEDs therapy is 0.3–0.8 % [6, 24]. Studies about the frequency and the overall trend of seizures in pregnancy are conflicting: some state there is a reduction (22.7 %), others the opposite (24.1 %) and in many cases (53.2 %) no changes are observed [25]. Seizures can cause bradycardia and foetal death [26] in utero due to status epilepticus [27, 28]. Therefore, it is advisable to ensure a good control of seizures during pregnancy [29]. The risk of seizures during labour is low, but it is sufficient to justify the recommendation that the delivery should take place in a department of obstetrics with services for maternal and neonatal resuscitation and treatment of maternal seizures [1]. Seizures can be severe during labour, although they can rarely cause foetal asphyxia and affect active collaboration of the mother during childbirth. Planning a caesarean section is needed in some cases only: in patients who have a history of frequent seizures, because they show a higher risk of protracted crises before and during labour. On the other hand, in some cases, there are specific indications for vaginal delivery [30]. There are no documented contraindications for epidural analgesia and prostaglandins for local use and for induction of labour in case of therapeutic abortion. As widely reviewed by Borthen [31], recent studies strongly indicate an association between AEDs use, and complications during pregnancy and labour. In particular, women with epilepsy seem to have a higher risk of preeclampsia and gestational hypertension [31], bleeding in pregnancy [32], caesarean delivery [33, 34], excessive bleeding post-partum, preterm birth and small for gestational age child [35, 36]. It is unclear whether the increased risk of complications is due to epilepsy per se, AEDs use, or combination of both. Moreover, we should consider that several conditions, such as advanced maternal age, low parity, low Bishop score and low duration of labour are at a higher risk of caesarean section [37, 38], independently from the epileptic status. As far as neonatal outcomes are concerned, there have been identified small for gestational age children (SGA) [39, 40], low Apgar score at 1 min [34], deterioration of potential long-term cognition [41] and perinatal death [42].
Modification of antiepileptic drugs’ pharmacokinetics during pregnancy
The physiological changes occurring in pregnancy may also significantly alter the absorption, distribution, metabolism and excretion of AEDs and, consequently, their plasma concentration [43]. Usually, AEDs concentration begins to decline significantly from the first to the third quarter of pregnancy. The influence of pregnancy on emotional and behavioural factors must be taken into account, as well as physical factors, such as emesis and pelvic distortion [44]. Thus, plasma concentration of AEDs, in particular those metabolized through glucuronidation, decreases during pregnancy [44]. This often happens to phenytoin, frequently to phenobarbital, carbamazepine and valproic acid [45]. Primidone’s plasma concentration remains stable, whereas phenobarbital’s (which derives from primidone) one is considerably reduced, mostly after childbirth [46]. The kinetics of new antiepileptic drugs is still poorly understood. Lamotrigine is the most studied: its concentration decreases during pregnancy, but sharply increases after childbirth [44, 45]. The mechanisms involved to explain these changes include reduction of the gastric motility [16, 18] and the concentration of albumin [47], increase of the plasma volume [11, 12], increase or decrease of the metabolic capacity and increase of the renal blood flow [13]. The behaviour of drugs with high protein binding, especially phenytoin and valproic acid, requires some additional considerations [45, 46]. Lower concentration of albumin that occurs during pregnancy leads to a reduction in the percentage of drug molecules bound to proteins. In response to a decrease of the total concentration, there is therefore a reduction of the pharmacologically active percentage of the drug (the unbound fraction) [44, 46, 47]. This phenomenon is particularly pronounced for valproic acid, the concentration of which is generally stable during pregnancy. In this and in other cases, monitoring the sole increase of the plasma concentration can be misleading [45, 48]. Furthermore, women with epilepsy have reproductive endocrine disorders [49] which include abnormalities of the menstrual cycle, anovulatory menstrual cycles [50, 51], which have been linked to AEDs treatment [50, 52]. They alter luteinizing hormone (LH) release, in response to gonadotropin-releasing hormone (GnRH) stimulation (Fig. 1) [53–56]. Some AEDs (phenobarbital, primidone, phenytoin, carbamazepine) are able to cause an increase of the activity of hepatic enzymes, whereas another AED (valproic acid) shows the very opposite action. The induction of CYP450 enzyme causes the reduction of serum concentrations of estradiol, testosterone, dehydroepiandrostenedione and increases the concentration of sex hormone-binding globulin (SHBG) [49, 55]. Therefore, clinical evaluation of plasma levels of antiepileptic drugs during and after pregnancy is important. Most cases do not require dose adjustment of AEDs, except those cases that show changes in the clinical situation.
Influence of the antiepileptic drugs on hypothalamic–pituitary axis. The antiepileptic drugs (AEDs) could interfere on hypothalamic–pituitary pathways: LH (luteinizing hormone) releasing is altered in response to GnRH (gonadotropin-releasing hormone); some AEDs such as carbamazepine, phenytoin and phenobarbital induce the hepatic cytochrome P450 enzyme (CYP450) that causes a decrease in serum estradiol concentrations, testosterone and dihydroepiandrostenedione, while valproate inhibits CYP450 causing increased levels of adrenal and gonadal androgens. CBZ carbamazepine, PHB phenobarbital, PHT phenytoin, VPA valproate, CYP cytochrome P450 isozyme
Risk of congenital malformations
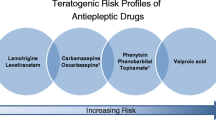
The estimated incidence of congenital malformations in the general population is 2–3 %, whereas for women undergoing AEDs treatment it is −6 %. It is therefore clear that the incidence is doubled in the latter [57, 58]. Recent studies have investigated three possible causes for this: direct toxicity of AEDs, probable folate deficiency caused by AEDs themselves, and reduced enzyme activity of epoxide hydrolase caused by genetic alterations [59]. It has been reported that genetic differences in folate metabolism may explain the increased risk of congenital abnormalities, particularly neural tube defects in children of women with epilepsy treated with AEDs [60, 61]. A study [62] shows that commonly used anticonvulsants such as carbamazepine, phenytoin and sodium valproate, used during pregnancy, showed incidents of interference with folic acid metabolism, thus causing foetal anticonvulsant syndrome. The mutation in the methylenetetrahydrofolate gene (MTHFR which is involved in the processing of 5–10 methylenetetrahydrofolate to 5-methyltetrahydrofolate) in mothers treated with sodium valproate, phenytoin or carbamazepine in pregnancy is associated with foetal anticonvulsant syndrome [63, 64]. Moreover, the skewed distribution of genotypes in affected children probably reflects the association of foetal anticonvulsant syndrome with the maternal genotype [65]. The old generation of antiepileptic drugs such as benzodiazepines, phenytoin, carbamazepine, phenobarbital, and valproic acid is teratogenic. Minor congenital malformations, such as facial dysmorphism and anomalies occur in 6–20 % of infants exposed to AEDs in utero [66], about twice as the percentage that occurs in general population. MCM (major congenital malformations) such as cleft lip and cleft palate, heart (atrial septal defect, tetralogy of Fallot, ventricular septal defect, aortic coarctation, patent ductus arteriosus, and pulmonary stenosis) and urogenital defects are estimated to be about 6 % of infants born to mothers treated with antiepileptic drugs, compared with 2–4 % of the general population [67]. Treatment with valproic acid during the first month of pregnancy is associated with neural tube defects (spina bifida and anencephaly) which have been estimated to be about 1–2 % [68], while treatment with carbamazepine showed similar malformations with an incidence of 0.5–1 % [69]. Holmes et al. [70] confirmed these results, showing increased rates of MCM; the combined frequency of embryopathy was 20.6 % in women treated with AEDs, whereas it was 8.5 % in the control group, and this rate increased from 20.6 to 28.0 % with AEDs in polytherapy There is little information regarding the teratogenicity of new antiepileptic drugs. The association between exposure to antiepileptic drugs in pregnancy and congenital defects has been the subject of intense studies for more than thirty years [71–75]. Nevertheless, the results of the studies published so far do not allow us to define the actual risk of malformations in children of epileptic mothers, or to identify safer drugs [76]. Studies carried out in relatively large populations, but with low statistical power, show a trend to greater teratogenicity of valproic acid compared with other antiepileptic drugs. Several studies, on the other hand, suggest that the teratogenicity of valproic acid may in part be due to genetic factors and that, at least in some cases, finding the presence of neural tube defects in previous pregnancies significantly increases the risk of birth defects in subsequent pregnancies [68]. It is not currently possible to analyse the outcomes of pregnancies in women without a family history of malformations, and to determine the actual relationship between the exposure to valproic acid and the incidence of malformation [64–68, 70, 76]. As far as the use of polytherapy is concerned, many studies [64–68, 70, 76] suggest that the number of AEDs used by the mother is directly correlated with the risk of MCM. It is likely that the teratogenic risk depends on the type of the combination therapy, therefore any association should be evaluated separately. Studies of UK Epilepsy and Pregnancy Register and Lamictal International Registry showed that the treatment in polytherapy containing valproate may increase the rate of malformations [77]. These data are in agreement with several other studies [78, 79]. The result of these data indicates that polytherapy in pregnancy is not necessarily risky, as it is the type of drugs chosen. Therefore, it is important to pay attention to which drug to prescribe in polytherapy in a pregnant woman [78, 80]. For example, although Valproate alone generates an increased risk of malformations, it has a even more increased risk when used in combination with another AED [77]. These results demonstrate that pregnant women who require treatment with AEDs in monotherapy are exposed to a lower risk than women who practice a polytherapy with AEDs. As far as the role of the dose of the drugs administered to the mother is concerned, the scientific evidence is conflicting. According to some Authors, high concentrations of phenobarbital and phenytoin increase the risk of malformations [81, 82], while other Authors do not consider it to be a relevant factor [83]. Only for valproic acid, data are more consistent, indicating the existence of a relationship between dose and teratogenicity [82]. The study by Mawer et al. [41] showed no statistically significant differences of MCM compared to controls, while the frequency of congenital malformations had a higher prevalence (6.6 %), with a peak (13.7 %) in women with epilepsy treated with valproate in polytherapy. The perspective is not encouraging: if the conventional studies have not yet defined the teratogenic risk of the older antiepileptic drugs (when they are the only alternative therapy), it is unlikely that we can get clear guidance on the new AEDs, generally less used in treatment of women of childbearing age [81]. Furthermore, the infants whose mothers had a history of epilepsy but took no anticonvulsant drugs during pregnancy did not have a higher frequency of those abnormalities than the control infants [70]. Finally, exposure to AEDs in utero could also impair cognitive function later in life. In this regard, valproate has been shown to have the most deleterious consequences on cognition, whereas human studies suggest a low risk for cognitive deficits with lamotrigine, levetiracetam, and carbamazepine [84].
The introduction of new antiepileptic drugs has further complicated the therapeutic strategy in women of childbearing age, as it is not possible to know the teratogenic risk related to the new molecules compared to conventional antiepileptic drugs; consequently, in the absence of reliable data, they should be considered potentially teratogenic. Many studies (Table 1), with rigorous methods and numerous cases, have sought to determine the incidence of MCM for every single drug.
Recommendations and prevention
Epilepsy is not a contraindication for pregnancy. Over 95 % of women with epilepsy will have a term pregnancy and healthy children [85]. In the first trimester of pregnancy, it is recommended to avoid (if possible) valproate, phenytoin, phenobarbital and valproate in polytherapy with other AEDs [32, 41]. The use of contraceptives is important for women of childbearing age, to increase the percentage of planned pregnancies and therefore minimize the risk of complications. [86]. This should include the information about the risks associated with epilepsy and pregnancy, potential interactions with oral contraceptive therapy and recommend the integration of drugs with particular reference to folate [85–87].
Monitoring of pregnancy, childbirth and post-partum
Pregnancy is a unique condition and AEDs can interact with pre-existing conditions, genetic variables and environmental factors. The focus on women with epilepsy of childbearing age should start before the occurrence of pregnancy [88]. Indeed, pregnancy can markedly affect the pharmacokinetics of several AEDs, and dose adjustment is often necessary during pregnancy to maintain the control of the crisis [89]. Preconception counselling should include patient education to ensure a clear understanding of the risks from uncontrolled seizures and possible teratogenicity of AEDs [88, 90]. Genetic counselling should be considered, especially in case of maternal/paternal idiopathic epilepsy and a positive family history of epilepsy [1]. A study by Kaneko et al. [80] found that the incidence of birth defects is related to the number of drugs and to their dosing, establishing that the incidence of birth defects decreases with AEDs in monotherapy, given in doses that must not exceed 1000 mg/day (plasma concentrations not exceeding 70 microg/ml). To prevent seizures during labour, crisis control should be achieved during the third quarter of pregnancy by establishing the effective dose with the minimum dosage. Folic acid 0.4–0.8 mg/day [59, 60, 92] during the preconception period and 4–5 mg/day afterwards [1, 88, 91] should be given 3 months before conception and during the first trimester of pregnancy, to avoid malformations due to folate deficit. From 14 to 18 weeks of gestation, serum alpha-fetoprotein should be evaluated. Pregnant women, who take AEDs, must undergo ultrasound high-resolution screening for structural abnormalities at 18–20 weeks of gestation, by an appropriately trained sonographer [1, 88, 92]. Monitoring of serum concentrations of the drug must be performed monthly [91]. Vitamin K prophylaxis is recommended starting at 36 weeks of gestation, to limit the effects induced by AEDs on microsomal enzymes that degrade foetal vitamin K and inhibit the γ-carboxy-glutamic acid for the precursors of coagulation factors II, VII, IX and X [88, 93, 94]. In this regard, Pack [95] recommends administration of vitamin K, at a dose of 10 mg/day, during the last month; conversely other Authors [1] do not recommend vitamin K supplementation to the mother, but to the newborn. Breastfeeding is generally possible because AEDs’ plasma concentrations are low and no adverse effects have been reported [88, 96, 97]. Special attention should be taken if the mothers are treated with ethosuximide, phenobarbital or primidone, which may induce sedation and lethargy in newborns [98].
Counselling
All epileptic women of childbearing age should have adequate information during preconception; clinical history must be assessed; both the type of AEDs and the dosage must be reassessed in relation to the clinical, obstetric and physiological situation [89]. The success of the counselling will be determined by a combination of care, collaboration of the patient and social context; a proper planning can reduce the adverse outcomes of pregnancy [99]. Planning must pay attention to the safety of the therapy during pregnancy, the potential neonatal, labour and delivery complications, and the management of neonatal and post-partum periods [100]. The counselling team, during preconception and pregnancy, should consist of a general practitioner, a neurologist, and an obstetrician, whereas during the time of childbirth and the post-partum there should also be an anesthesiologist, a neonatologist and a psychiatrist [101, 102]. The evidence suggests that adequate information and planning of pregnancy in women with epilepsy can help to minimize the risks associated with symptoms of the disease and the toxicity of AEDs (Table 2; Fig. 2). Finally, it is extremely important for the clinician to involve the patient in clinical decision-making, to make decisions together using the best evidence [103]. As far as shared decision-making is concerned, although little evidence is available on this matter in literature, we agree on using this protocol whenever more than one option is possible.
Discussion
The teratogenicity of AEDs remains nowadays a problem to investigate. In literature, there are indeed many studies that have reported contradictory results. Among these studies, few Authors do not consider genetic predisposition as a cause of foetal malformations, regardless of the drug or its possible effect on the genetic background. The quantitative and qualitative analysis of published studies does not define the degree of the risk of malformations in children of mothers with epilepsy, neither identifies a scale of risk of the drugs. There were reports of birth defects in mothers treated with AEDs (especially regarding neural tube defects), but with significant rates of other musculoskeletal, cardiac, urogenital abnormalities, craniofacial dysmorphic features, hypospadias, cleft lip and palate, microcephaly. There is evidence of a “phenytoin syndrome” with growth retardation, microcephaly, craniofacial dysmorphism, hypoplastic nails and distal phalanges, cone epiphyses. The heterogeneity of data in literature and the absence of conclusive information is fundamentally dependent on methodological issues, most notably that of the sample size. Epilepsy is not a unique condition, possible drug associations are numerous, as well as potential risk factors. It is primarily for this reason that none of the published studies has in fact an adequate statistical power. These considerations have led researchers from different countries to work together to achieve prospective observational records, considered by many to be the only adequate means to collect an adequate sample within a reasonable time to evaluate the teratogenic risk of individual drugs and associations of them. Nevertheless, obstetric management of epileptic women could be influenced by several different conditions, such as the impact that some supplementations [104–107] or comorbidities [108, 109] may have on the status of the disease, necessity of pharmacological treatment [110, 111] or other obstetric interventions [112, 113] and drugs adsorption starting from the periconceptional period until post-partum. In this regard, each case should be managed considering all these variables, possibly performing a tailored treatment.
Conclusions
Even considering that this report is a narrative (non-systematic) review of the literature data, it allows us to state that certainly epilepsy is not a contraindication for pregnancy. In fact, over 95 % of women treated with AEDs during pregnancy undergo safe pregnancy and delivery. The problems related to antiepileptic therapy and the possibilities of prenatal diagnosis should be discussed thoroughly with the patient, if possible before pregnancy, considering individual circumstances, desire to have children, severity of epilepsy, family history of congenital malformations and all other potential risk factors. Finally, considering that the quantitative and qualitative analysis of published studies does not define the degree of the risk of malformations in children from mothers with epilepsy, neither identifies a scale of risk of the drugs, we strongly encourage future studies, based on large cohorts and with rigorous methodology, to improve our current knowledge of the topic.
References
Care NCC for P (2004) The diagnosis and management of the epilepsies in adults and children in primary and secondary care
Ngugi AK, Kariuki SM, Bottomley C et al (2011) Incidence of epilepsy: a systematic review and meta-analysis. Neurology 77:1005–1012. doi:10.1212/WNL.0b013e31822cfc90
Hirtz D, Thurman DJ, Gwinn-Hardy K et al (2007) How common are the “common” neurologic disorders? Neurology 68:326–337. doi:10.1212/01.wnl.0000252807.38124.a3
Maiga Y, Napon C, Kuate Tegueu C et al (2010) Epilepsy and women’s life: particularities of their management. Literature review. Mali Med 25:1–9
Fisher RS, Van Emde Boas W, Blume W et al (2005) Epileptic seizures and epilepsy: definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia 46:470–472. doi:10.1111/j.0013-9580.2005.66104.x
Hauser WA, Annegers JF, Rocca WA (1996) Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc 71:576–586. doi:10.4065/71.6.576
Berg AT, Shinnar S (1991) The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology 41:965–972. doi:10.1212/WNL.41.7.965
Hauser WA, Rich SS, Lee JR et al (1998) Risk of recurrent seizures after two unprovoked seizures. N Engl J Med 338:429–434. doi:10.1097/00007611-199806000-00028
Robson S, Hunter S, Boys R, Dunlop W (1989) Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 256:H1060–H1065
Clark SL, Cotton DB, Lee W et al (1989) Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol 161:1439–1442. doi:10.1097/00132586-199102000-00026
Messmer K, Kemming G (2006) Clinical hemodilution. Blood Substit. pp 178–187
American College of Obstetricians and Gynecologists (2008) ACOG practice bulletin no. 95: anemia in pregnancy. Obs Gynecol 112:201–207. doi:10.1097/AOG.0b013e3181809c0d
Davison JM, Dunlop W (1980) Renal hemodynamics and tubular function in normal human pregnancy. Kidney Int 18:152–161
Hegewald MJ, Crapo RO (2011) Respiratory physiology in pregnancy. Clin Chest Med 32:1–13. doi:10.1016/j.ccm.2010.11.001
McAuliffe F, Kametas N, Costello J et al (2002) Respiratory function in singleton and twin pregnancy. BJOG Int J Obstet Gynaecol 109:765–769. doi:10.1111/j.1471-0528.2002.01515.x
Fisher RS, Roberts GS, Grabowski CJ, Cohen S (1978) Altered lower esophageal sphincter function during early pregnancy. Gastroenterology 74:1233–1237
Vellacott ID, Cooke EJ, James CE (1988) Nausea and vomiting in early pregnancy. Int J Gynaecol Obstet 27:57–62. doi:10.1016/0020-7292(88)90088-4
Parry E, Shields R, Turnbull AC (1970) The effect of pregnancy on the colonic absorption of sodium, potassium and water. J Obstet Gynaecol Br Commonw 77:616–619. doi:10.1111/j.1471-0528.1970.tb03579.x
Everson GT (1998) Liver problems in pregnancy: distinguishing normal from abnormal hepatic changes. Medscape Womens Health 3:3
Celik H, Tosun M, Cetinkaya MB et al (2009) Markedly elevated serum alkaline phosphatase level in an uncomplicated pregnancy. J Matern Fetal Neonatal Med 22:705–707. doi:10.1080/14767050802702323
Chrousos GP (1995) The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332:1351–1362. doi:10.1056/NEJM199505183322008
Petraglia F, Potter E, Cameron VA et al (1993) Corticotropin-releasing factor-binding protein is produced by human placenta and intrauterine tissues. J Clin Endocrinol Metab 77:919–924
Petraglia F, Florio P, Nappi C, Genazzani AR (1996) Peptide signaling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocr Rev 17:156–186. doi:10.1210/er.17.2.156
Borthen I, Eide MG, Veiby G et al (2009) Complications during pregnancy in women with epilepsy: population-based cohort study. BJOG Int J Obstet Gynaecol 116:1736–1742. doi:10.1111/j.1471-0528.2009.02354.x
Rating D, Nau H, Jäger-Roman E et al (1982) Teratogenic and pharmacokinetic studies of primidone during pregnancy and in the offspring of epileptic women. Acta Paediatr Scand 71:301–311
Teramo K, Hiilesmaa V, Bardy A, Saarikoski S (1979) Fetal heart rate during a maternal grand mal epileptic seizure. J Perinat Med 7:3–6. doi:10.1515/jpme.1979.7.1.3
Bardy AH, Hiilesmaa VK, Teramo K, Granström ML (1981) Teratogenic risks of antiepileptic drugs. Br Med J (Clin Res Ed) 283:1405–1406. doi:10.1136/bmj.283.6303.1405-d
Battino D, Tomson T, Bonizzoni E et al (2013) Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia 54:1621–1627. doi:10.1111/epi.12302
Olafsson E, Hallgrimsson JT, Hauser WA et al (1998) Pregnancies of women with epilepsy: a population-based study in Iceland. Epilepsia 39:887–892
Richmond JR, Krishnamoorthy P, Andermann E, Benjamin A (2004) Epilepsy and pregnancy: an obstetric perspective. Am J Obstet Gynecol 190:371–379. doi:10.1016/j.ajog.2003.09.020
Borthen I, Eide MG, Daltveit AK, Gilhus NE (2011) Obstetric outcome in women with epilepsy: a hospital-based, retrospective study. BJOG Int J Obstet Gynaecol 118:956–965. doi:10.1111/j.1471-0528.2011.03004.x
Harden CL, Meador KJ, Pennell PB et al (2009) Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): II. Teratogenesis and perinatal outcomes. Epilepsia 50:1237–1246. doi:10.1111/j.1528-1167.2009.02128.x
Kelly VM, Nelson LM, Chakravarty EF (2009) Obstetric outcomes in women with multiple sclerosis and epilepsy. Neurology 73:1831–1836. doi:10.1212/WNL.0b013e3181c3f27d
Veiby G, Daltveit AK, Engelsen BA, Gilhus NE (2009) Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia 50:2130–2139. doi:10.1111/j.1528-1167.2009.02147.x
Viinikainen K, Heinonen S, Eriksson K, Kälviäinen R (2006) Community-based, prospective, controlled study of obstetric and neonatal outcome of 179 pregnancies in women with epilepsy. Epilepsia 47:186–192. doi:10.1111/j.1528-1167.2006.00386.x
Hvas CL, Henriksen TB, Ostergaard JR, Dam M (2000) Epilepsy and pregnancy: effect of antiepileptic drugs and lifestyle on birthweight. BJOG 107:896–902
Gerli S, Favilli A, Giordano C et al (2013) Single indications of induction of labor with prostaglandins and risk of cesarean delivery: a retrospective cohort study. J Obstet Gynaecol Res 39:926–931. doi:10.1111/jog.12000
Favilli A, Acanfora MM, Bini V et al (2013) Single indication of labor induction with prostaglandins: is advanced maternal age a risk factor for cesarean section? A matched retrospective cohort study. J Matern Fetal Neonatal Med 26:665–668. doi:10.3109/14767058.2012.746658
Zahn CA, Morrell MJ, Collins SD et al (1998) Management issues for women with epilepsy: a review of the literature. Neurology 51:949–956. doi:10.1212/WNL.51.4.949
Lundgren EM, Tuvemo T (2008) Effects of being born small for gestational age on long-term intellectual performance. Best Pract Res Clin Endocrinol Metab 22:477–488. doi:10.1016/j.beem.2008.01.014
Mawer G, Briggs M, Baker GA et al (2010) Pregnancy with epilepsy: obstetric and neonatal outcome of a controlled study. Seizure 19:112–119. doi:10.1016/j.seizure.2009.11.008
Meador KJ, Pennell PB, Harden CL et al (2008) Pregnancy registries in epilepsy: a consensus statement on health outcomes. Neurology 71:1109–1117. doi:10.1212/01.wnl.0000316199.92256.af
Patsalos PN, Berry DJ, Bourgeois BFD et al (2008) Antiepileptic drugs—best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE commission on therapeutic strategies. Epilepsia 49:1239–1276. doi:10.1111/j.1528-1167.2008.01561.x
Brodtkorb E, Reimers A (2008) Seizure control and pharmacokinetics of antiepileptic drugs in pregnant women with epilepsy. Seizure 17:160–165. doi:10.1016/j.seizure.2007.11.015
Pennell PB (2003) Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology 61:S35–S42. doi:10.1212/WNL.61.6_suppl_2.S35
Battino D, Avanzini G, Bossi L et al (1983) Plasma levels of primidone and its metabolite phenobarbital: effect of age and associated therapy. Ther Drug Monit 5:73–79. doi:10.1097/00007691-198303000-00006
Costantine MM (2014) Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. doi:10.3389/fphar.2014.00065
Reisinger TL, Newman M, Loring DW et al (2013) Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav 29:13–18. doi:10.1016/j.yebeh.2013.06.026
Morrell MJ, Giudice L, Flynn KL et al (2002) Predictors of ovulatory failure in women with epilepsy. Ann Neurol 52:704–711. doi:10.1002/ana.10391
Drislane FW, Coleman AE, Schomer DL et al (1994) Altered pulsatile secretion of luteinizing hormone in women with epilepsy. Neurology 44:306–310. doi:10.1212/WNL.44.2.306
Meo R, Bilo L, Nappi C et al (1993) Derangement of the hypothalamic GnRH pulse generator in women with epilepsy. Seizure 2:241–252. doi:10.1016/S1059-1311(05)80134-7
Murialdo G, Galimberti CA, Magri F et al (1997) Menstrual cycle and ovary alterations in women with epilepsy on antiepileptic therapy. J Endocrinol Invest 20:519–526
Verrotti A, D’Egidio C, Mohn A et al (2011) Antiepileptic drugs, sex hormones, and PCOS. Epilepsia 52:199–211. doi:10.1111/j.1528-1167.2010.02897.x
Herzog AG, Seibel MM, Schomer DL et al (1986) Reproductive endocrine disorders in men with partial seizures of temporal lobe origin. Arch Neurol 43:347–350. doi:10.1001/archneur.1986.00520040035015
Morrell MJ, Flynn KL, Seale CG et al (2001) Reproductive dysfunction in women with epilepsy: antiepileptic drug effects on sex-steroid hormones. CNS Spectr 6(771–772):783–786
Fenwick PB, Toone BK, Wheeler MJ et al (1985) Sexual behaviour in a centre for epilepsy. Acta Neurol Scand 71:428–435
Thomas SV, Nair RR, Jose M, Sarma PS (2009) Risk of major congenital malformations in the offsprings of women with epilepsy is not related to family history. Epilepsy Res 83:52–57. doi:10.1016/j.eplepsyres.2008.09.002
Wide K, Winbladh B, Källén B (2004) Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr 93:174–176. doi:10.1080/08035250310021118
Kelly TE (1984) Teratogenicity of anticonvulsant drugs. I: review of the literature. Am J Med Genet 19:413–434
Daly LE, Kirke PN, Molloy A et al (1995) Folate levels and neural tube defects. Implications for prevention. JAMA 274:1698–1702. doi:10.1001/jama.274.21.1698
Czeizel AE, Dudás I, Paput L, Bánhidy F (2011) Prevention of neural-tube defects with periconceptional folic acid, methylfolate, or multivitamins? Ann Nutr Metab 58:263–271. doi:10.1159/000330776
Kishi T, Fujita N, Eguchi TA, Ueda K (1997) Mechanism for reduction of serum folate by antiepileptic drugs during prolonged therapy. J Neurol Sci 145:109–112. doi:10.1016/S0022-510X(96)00256-0
Yoo JH, Hong SB (1999) A common mutation in the methylenetetrahydrofolate reductase gene is a determinant of hyperhomocysteinemia in epileptic patients receiving anticonvulsants. Metabolism 48:1047–1051. doi:10.1016/S0026-0495(99)90204-4
Verrotti A, Pascarella R, Trotta D et al (2000) Hyperhomocysteinemia in children treated with sodium valproate and carbamazepine. Epilepsy Res 41:253–257. doi:10.1016/S0920-1211(00)00150-9
Dean JC, Moore SJ, Osborne A et al (1999) Fetal anticonvulsant syndrome and mutation in the maternal MTHFR gene. Clin Genet 56:216–220. doi:10.1034/j.1399-0004.1999.560306.x
Gaily E, Granström ML, Hiilesmaa V, Bardy A (1988) Minor anomalies in offspring of epileptic mothers. J Pediatr 112:520–529
Koch S, Lösche G, Jager-Romän E et al (1992) Major and minor birth malformations and antiepileptic drugs. Neurology 42:83–88
Omtzigt JG, Los FJ, Grobbee DE et al (1992) The risk of spina bifida aperta after first-trimester exposure to valproate in a prenatal cohort. Neurology 42:119–125
Gilboa SM, Broussard CS, Devine OJ et al (2011) Influencing clinical practice regarding the use of antiepileptic medications during pregnancy: modeling the potential impact on the prevalences of spina bifida and cleft palate in the United States. Am J Med Genet Part C Semin Med Genet 157:234–246. doi:10.1002/ajmg.c.30306
Holmes LB, Harvey EA, Coull BA et al (2001) The teratogenicity of anticonvulsant drugs. N Engl J Med 344:1132–1138. doi:10.1097/00006254-200109000-00006
Matalon S, Schechtman S, Goldzweig G, Ornoy A (2002) The teratogenic effect of carbamazepine: a meta-analysis of 1255 exposures. Reprod Toxicol 16:9–17. doi:10.1016/S0890-6238(01)00199-X
Artama M, Auvinen A, Raudaskoski T et al (2005) Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology 64:1874–1878. doi:10.1212/01.WNL.0000163771.96962.1F
Hernandez-Diaz S, Smith CR, Shen A et al (2011) Comparative safety of anticonvulsants during pregnancy: seizures or major malformations. Pharmacoepidemiol Drug Saf 20:S11
Hunt SJ, Craig JJ, Morrow JI (2009) Increased frequency of isolated cleft palate in infants exposed to lamotrigine during pregnancy. Neurology 72:1108. doi:10.1212/01.wnl.0000346463.44116.56
Holmes LB (2011) Controls for pregnancy registries. Birth Defects Res Part A Clin Mol Teratol 91:386
Tomson T, Perucca E, Battino D (2004) Navigating toward fetal and maternal health: the challenge of treating epilepsy in pregnancy. Epilepsia 45:1171–1175. doi:10.1111/j.0013-9580.2004.15104.x
Morrow JI, Russell AJ, Irwin B et al (2004) The safety of antiepileptic drugs in pregnancy: results of the UK epilepsy and pregnancy register. Epilepsia 45(Suppl 3):57
Dansky LV, Andermann E, Rosenblatt D, Sherwin AL (1987) Anticonvulsants, folate levels, and pregnancy outcome: a prospective study. Ann Neurol 21:176–182. doi:10.1002/ana.410210210
Cunnington MC, Weil JG, Messenheimer JA et al (2011) Final results from 18 years of the international lamotrigine pregnancy registry. Neurology 76:1817–1823. doi:10.1212/WNL.0b013e31821ccd18
Kaneko S, Battino D, Andermann E et al (1999) Congenital malformations due to antiepileptic drugs. Epilepsy Res 33:145–158. doi:10.1016/S0920-1211(98)00084-9
Kaaja E, Kaaja R, Hiilesmaa V (2003) Major malformations in offspring of women with epilepsy. Neurology 60:575–579. doi:10.1212/WNL.61.11.1631-a
Vajda FJE, Hitchcock A, Graham J et al (2006) Foetal malformations and seizure control: 52 months data of the Australian pregnancy registry. Eur J Neurol 13:645–654. doi:10.1111/j.1468-1331.2006.01359.x
Barrett C, Richens A (2003) Epilepsy and pregnancy: report of an epilepsy research foundation workshop. Epilepsy Res 52:147–187
Inoyama K, Meador KJ (2015) Cognitive outcomes of prenatal antiepileptic drug exposure. Epilepsy Res 114:89–97. doi:10.1016/j.eplepsyres.2015.04.016
Nakken KO, Lillestølen KM, Brodtkorb E et al (2014) Antiepileptika og medfødte misdannelser. Tidsskr den Nor Laegeforening 134:1239–1242. doi:10.4045/tidsskr.13.1349
Sabers A (2009) Influences on seizure activity in pregnant women with epilepsy. Epilepsy Behav 15:230–234. doi:10.1016/j.yebeh.2009.03.031
Hui ACF, Yeung HM (2001) Avoiding pitfalls in the management of epilepsy. Hong Kong Pract 23:246–250
Crawford P (2005) Best practice guidelines for the management of women with epilepsy. Epilepsia 46:117–124. doi:10.1111/j.1528-1167.2005.00323.x
Perucca E, Battino D, Tomson T (2014) Gender issues in antiepileptic drug treatment. Neurobiol Dis. doi:10.1016/j.nbd.2014.05.011
Bajcar JM, Kennie N, Einarson TR (2005) Collaborative medication management in a team-based primary care practice: an explanatory conceptual framework. Res Soc Adm Pharm 1:408–429. doi:10.1016/j.sapharm.2005.06.003
Nulman I, Laslo D, Koren G (1999) Treatment of epilepsy in pregnancy. Drugs 57:535–544. doi:10.2165/00003495-199957040-00006
Luef G (2009) Female issues in epilepsy: a critical review. Epilepsy Behav 15:78–82. doi:10.1016/j.yebeh.2009.02.023
Choulika S, Grabowski E, Holmes LB (2004) Is antenatal vitamin K prophylaxis needed for pregnant women taking anticonvulsants? Am J Obstet Gynecol 190:882–883. doi:10.1016/j.ajog.2004.01.041
Kaaja E, Kaaja R, Matila R, Hiilesmaa V (2002) Enzyme-inducing antiepileptic drugs in pregnancy and the risk of bleeding in the neonate. Neurology 58:549–553. doi:10.1212/WNL.58.4.549
Pack AM (2006) Therapy insight: clinical management of pregnant women with epilepsy. Nat Clin Pract Neurol 2:190–200. doi:10.1038/ncpneuro0153
Öhman I, Vitols S, Luef G et al (2002) Topiramate kinetics during delivery, lactation, and in the neonate: preliminary observations. Epilepsia 43:1157–1160. doi:10.1046/j.1528-1157.2002.12502.x
Johannessen SI, Helde G, Brodtkorb E (2005) Levetiracetam concentrations in serum and in breast milk at birth and during lactation. Epilepsia 46:775–777. doi:10.1111/j.1528-1167.2005.54804.x
Öhman I, Vitols S, Tomson T (2005) Pharmacokinetics of gabapentin during delivery, in the neonatal period, and lactation: does a fetal accumulation occur during pregnancy? Epilepsia 46:1621–1624. doi:10.1111/j.1528-1167.2005.00251.x
Pashley S, O’Donoghue MF (2009) The safety of anti-epileptic drug regimens: a qualitative study of factors determining the success of counselling women before conception. J Fam Plann Reprod Health Care 35:153–156. doi:10.1783/147118909788708002
McAuley JW, Patankar C, Lang C, Prasad M (2012) Evaluating the concerns of pregnant women with epilepsy: a focus group approach. Epilepsy Behav 24:246–248. doi:10.1016/j.yebeh.2012.03.014
Hart LA, Sibai BM (2013) Seizures in pregnancy: epilepsy, eclampsia, and stroke. Semin Perinatol 37:207–224. doi:10.1053/j.semperi.2013.04.001
Robinson JN, Cleary-Goldman J (2008) Chapter 16 management of epilepsy and pregnancy. An obstetrical perspective. Int Rev Neurobiol 83:273–282. doi:10.1016/S0074-7742(08)00016-0
Pickrell WO, Elwyn G, Smith PEM (2015) Shared decision-making in epilepsy management. Epilepsy Behav. doi:10.1016/j.yebeh.2015.01.033
Vitagliano A, Quaranta M, Noventa M, Gizzo S (2015) “Empiric” inositol supplementation in normal-weight non insulin resistant women with polycystic ovarian disease: from the absence of benefit to the potential adverse effects. Arch Gynecol Obstet 291:955–957. doi:10.1007/s00404-015-3662-9
Noventa M, Vitagliano A, Quaranta M et al (2015) Preventive and therapeutic role of dietary inositol supplementation in periconceptional period and during pregnancy: a summary of evidences and future applications. Reprod Sci. doi:10.1177/1933719115594018
Patrelli TS, Dall’Asta A, Gizzo S et al (2012) Calcium supplementation and prevention of preeclampsia: a meta-analysis. J Matern Neonatal Med 25:1–5. doi:10.3109/14767058.2012.715220
Cetin I, Berti C, Calabrese S (2009) Role of micronutrients in the periconceptional period. Hum Reprod Update 16:80–95. doi:10.1093/humupd/dmp025
Gizzo S, Patrelli TS, Rossanese M et al (2013) An update on diabetic women obstetrical outcomes linked to preconception and pregnancy glycemic profile: a systematic literature review. Sci World J 2013:254901. doi:10.1155/2013/254901
Gizzo S, Noventa M, Di Gangi S et al (2015) Could molecular assessment of calcium metabolism be a useful tool to early screen patients at risk for pre-eclampsia complicated pregnancy? Proposal and rationale. Clin Chem Lab Med 53:975–979. doi:10.1515/cclm-2014-0693
Gizzo S, Noventa M, Anis O et al (2014) Pharmacological anti-thrombotic prophylaxis after elective caesarean delivery in thrombophilia unscreened women: should maternal age have a role in decision making? J Perinat Med 42:339–347. doi:10.1515/jpm-2013-0160
Gizzo S, Patrelli TS, Di Gangi S et al (2013) Which uterotonic is better to prevent the postpartum hemorrhage? Latest news in terms of clinical efficacy, side effects, and contraindications: a systematic review. Reprod Sci 20:1011–1019. doi:10.1177/1933719112468951
Gizzo S, Noventa M, Fagherazzi S et al (2014) Update on best available options in obstetrics anaesthesia: perinatal outcomes, side effects and maternal satisfaction. Fifteen years systematic literature review. Arch Gynecol Obstet 290:21–34. doi:10.1007/s00404-014-3212-x
Gizzo S, Di Gangi S, Saccardi C et al (2012) Epidural analgesia during labor: impact on delivery outcome, neonatal well-being, and early breastfeeding. Breastfeed Med 7:262–268. doi:10.1089/bfm.2011.0099
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All Authors have no proprietary, financial, professional or other personal interest of any nature in any product, service or company. The Authors alone are responsible for the content and writing of the paper.
Rights and permissions
About this article
Cite this article
Laganà, A.S., Triolo, O., D’Amico, V. et al. Management of women with epilepsy: from preconception to post-partum. Arch Gynecol Obstet 293, 493–503 (2016). https://doi.org/10.1007/s00404-015-3968-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3968-7