Abstract
Background
Several studies have assessed the association between green and black tea consumption and the risk of endometrial cancer (EC) and have yielded inconsistent results.
Objective
The purpose of this meta-analysis is to systematically analyze the effect of green tea and black tea on EC risk.
Methods
PubMed, Embase, Cochrane Library and China Biological Medicine Database were searched through February 2, 2015 to identify studies that met pre-stated inclusion criteria. Overall relative risk (RR) was estimated based on the highest and lowest levels of green/black tea consumption. Dose–response relationships were evaluated with the data from categories of green/black tea intake in each study.
Results
For green tea, the summary RR indicated that the highest green tea consumption was associated with a reduced risk of EC (RR 0.78, 95 % CI 0.66–0.92). Furthermore, an increase in green tea consumption of one cup per day was associated with an 11 % decreased risk of developing EC. (RR 0.89, 95 % CI 0.84–0.94). For black tea, no statistically significant association was observed in the meta-analysis (highest versus non/lowest, RR 0.99, 95 % CI 0.79–1.23; increment of one cup/day, RR 0.99, 95 % CI 0.94–1.03). The power of the estimate of green tea and black tea with risk of EC was 84.33 and 5.07 %, respectively. The quality of evidence for the association between green and black tea with EC risk was moderate and very low, respectively.
Conclusions
The results from this meta-analysis indicate that green tea, but not black tea, may be related to a reduction of EC risk. Large population-based randomized controlled trials and large prospective cohort studies are required to obtain a definitive conclusion and determine the mechanisms underlying this association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, endometrial cancer (EC) has become one of the most common cancers in women, ranking as the fourth highest cause of female cancer-related deaths worldwide [1–3]. Interestingly, however, the incidence of endometrial cancer in developed countries is at least twofold greater than that observed in developing countries, with an age-standardized annual incidence rate (ASR) of 14.7 per 100,000 women and 5.5 per 100,000 women, respectively [4]. In the United States, it is estimated that approximately 54,870 women will be diagnosed in 2015 [5]. Tea is the second most popular beverage in the world [6] and originates from the leaves of the plant Camellia sinensis [7]. Different processing methods result in several types of tea. Of the total amount of tea consumed worldwide, 20 % is green tea (unfermented), 78 % is black tea (fermented) and 2 % is oolong tea (a kind of tea, which is considered to be about 50 % fermented) [7]. Tea contains a wide variety of antioxidants, such as flavonoids, catechins, thearubigins and theaflavins, some of which have been reported to exert potential anti-carcinogenic properties. Growing evidence from laboratory, epidemiologic, and human intervention studies suggests that consuming tea can exert preventive effects against many types of cancers [8].
To date, only one prior meta-analysis [9] has been conducted to address whether tea consumption is associated with EC risk. That meta-analysis combined data from five case–control studies and two cohort studies and found an inverse association between ever tea intake and non/very low tea consumption. Moreover, a stratified analysis by tea type showed that green tea, but not black tea, was beneficial in a lower EC risk. However, the previous meta-analysis only considered four and two studies on green tea and black tea, respectively. Since 2009, five studies have been published [10–14], and one study was not included in the previous meta-analysis [15]; given the new evidence, we conducted a meta-analysis of observational studies with the following objectives: (1) to update the epidemiology evidence on the association between green/black tea intake and the risk of EC; (2) to quantify the dose–response association between green/black tea consumption and EC; and (3) to evaluate the statistical power of the associations found in (1) and the overall strength of the evidence presented.
Materials and methods
A prospective protocol was registered on PROSPERO (http://www.crd.york.ac.uk/prospero/) with the identification number CRD42015017944. We attempted to report this meta-analysis in accordance with the MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines [16], which are included in the online supplement material.
Date source and search strategy
We searched PubMed, Embase, Cochrane Library and China Biological Medicine Database for studies published prior to February 2, 2015, that focused on describing the association between tea consumption and EC risk. The following key words were used in our search strategies: (“endometrial cancer” OR “endometrium cancer” OR “endometrial carcinoma” OR “endometrium carcinoma” OR “endometrial neoplasms” OR “endometrium neoplasms” OR “corpus uteri cancer” OR “corpus uteri neoplasm” OR “corpus uteri carcinoma” OR “uterus tumor” OR “uterine tumor”) AND (“Tea” OR “green tea” OR “black tea” OR “catechin” OR “theaflavin” OR “thearubigin”). In addition, we analyzed references from the original paper and review articles to identify further relevant studies. In cases of duplicated data, the most recent or most complete study was included in our analysis.
Study selection and data extraction
First, we performed an initial screening of the titles or abstracts, followed by a full-text review. Studies were considered eligible if they met the following criteria: (1) the experimental design included a case–control or cohort study; (2) the exposure of interest was tea intake; (3) the outcome of interest was endometrial cancer; and (4) the odds ratio (OR) or the relative risk (RR) and the corresponding 95 % confidence interval (CI) of endometrial cancer were provided for the highest and lowest levels and were adjusted for potential confounders. Two independent investigators (QZ and HL) conducted the study selection. For each included study, we extracted the following information: the first author’s last name, publication year, country of origin, study period, study design, tea intake categories, type of tea, the number of cases and controls in case–control studies or the number of cases and cohort size in cohort studies, ORs or RRs (with their 95 % confidence intervals) for each category of tea consumption and covariates used to adjust in the multivariate analysis. In cases in which the tea type was unspecified, the local tea drinking preference was used, including black tea for English-speaking countries [17] and green tea for Asian countries [18]. All data were retrieved by two independent investigators (QZ and JGZ), and any disagreements were resolved by consensus.
Quality assessment of study and evidence
The Newcastle–Ottawa Scale [19] (NOS) was used to assess the overall quality of the included studies. This scale ranged from 0 (poor) to 9 stars (excellent), and four stars were awarded for the selection of study participants, two stars for the comparability of studies based on the design or analysis, and three stars for the evaluation of exposure in case–control studies or ascertained outcomes in cohort studies. Studies with a score of 6 stars or greater were considered high quality. Two reviewers (HM and YM) evaluated the quality of evidence using the GRADE system [20] (using GRADE profiler 3.6.1). In this system, an observational study is initially assumed to contain low-quality evidence. The score can be downgraded for five reasons (i.e., risk of bias, inconsistency, indirectness, imprecision and publication bias) and upgraded for three reasons (i.e., a large effect, presentation of a dose–response gradient or plausible confounders that did not alter the apparent treatment effect). The evidence grades were divided into the following categories: (1) high, which indicated that further research was unlikely to alter confidence in the estimated effect; (2) moderate, which indicated that further research may significantly alter confidence in or change the estimated effect; (3) low, which indicated that further research was likely to significantly alter confidence in the effect estimate and to change the estimate; and (4) very low, which indicated that any effect estimate is uncertain.
Statistical analysis
Because the incidence of endometrial cancer is low [1], the OR is mathematically similar to RR [21]. Therefore, we reported all results as RR for simplicity. Associations between green and black tea intake with EC risk were quantified by comparing the highest and lowest (the referent) categories. For the dose–response meta-analysis, we used the “generalized least squares for trend estimation” method proposed by Greenland and Longnecker [22, 23], which considers the correlation of the log RR estimates across different exposures. This method requires knowledge of the cases and cohort size/number of controls of each category and the RR with its variance estimate for at least three quantitative exposure categories. For studies that did not provide the number of cases and cohort size/number of controls in each exposure category, we estimated the slopes using “variance-weighted least-squares regression model” [22]. Tea intake values were assigned as the medium provided by the original research. For studies that did not provide a median value, we used the midpoint for closed categories and similar amplitudes as the neighbouring open-ended categories. Statistical heterogeneity among studies was assessed using the χ2 test and was defined as a P value less than 0.10. Quantification of heterogeneity was made using the I 2 statistic [23], with low heterogeneity defined by I 2 values less than 50 % and values greater than 50 % indicative of high heterogeneity. The Begg rank correlation test [24] and Egger’s regression test [25] were used to detect publication bias, and publication bias was considered to exist when P < 0.10 [26]. We tried to explore the source of heterogeneity by subgroup analysis and conduct a sensitivity analysis to investigate the influence of a single study on the overall risk estimate by omitting one study in each turn. Power calculation was performed after the studies had been collected using the methodology described by Cafri et al. [27]. Details on the macro and SAS code used are included in the online supplement material. Statistical analyses were conducted in Stata Version 12.0 (StataCorp, College Station, TX, USA). A threshold of P < 0.05 was considered statistically significant, unless otherwise specified.
Results
Literature search
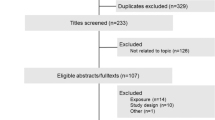
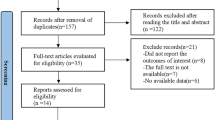
Figure 1 shows the results obtained in the literature research and selection. We identified 372 articles from PubMed, Embase, Cochrane Library and China Biological Medicine Database that were published prior to February 2, 2015, in our initial electronic search. A manual search of the reference lists yielded an additional 2 related papers. After excluding duplicate records and studies that did not fulfill our inclusion criteria, 21 articles remained. Of these, we excluded 8 studies as follows: 4 studies [28–31] did not provide data on tea intake, 2 studies [32, 33] did not provide information on endometrial cancer, 1 study [34] was a conference abstract and overlapped with a previous article, and 1 article was found to overlap [35]. In total, 13 studies [10–13, 15, 36–42] were shown to meet the inclusion criteria and were included in the meta-analysis. Among these articles, only one study [11] provided information for both type I and type II endometrial cancer and was thus considered two comparisons.
Study characteristics
Table 1 shows the characteristics extracted from the included studies. These studies were published between 1992 and 2014. Six studies were conducted primarily in the USA [11–14, 39, 42], five were conducted in Asia (China and Japan) [36–38, 40, 41], and two were conducted in Europe (Sweden and Italy) [10, 15]. Seven studies [14, 15, 36–39, 41] had a case–control design, comprising 3915 cases and 21880 controls; among them, four were population-based case–control studies, and the other three were hospital-based case–control studies. Six studies [10–13, 40, 42] had a cohort design and comprised 2151 cases and 267,885 participants. The number of participants ranged from 67,470 in the Nurses’ Health Study (NHS) [12] to 23,356 in the Iowa Women’s Health Study (IWHS) [11]. Seven studies used food-frequency questionnaires (FFQ) to assess tea intake, and the remaining studies used other questionnaires. In some case–control studies, the Body Mass Index (BMI) of case group was higher than that of control group. And many cases were ascertained through cancer registries (see online supplemental material). Ten comparisons focused on black tea intake [10–15, 39, 41, 42], and six comparisons [14, 36–38, 40, 41] focused on green tea. The NOS scores (Table 2) ranged from five to nine. Eight of the thirteen studies were determined to have relatively high quality (over 6 stars), and the mean NOS score was 7.08 stars (standard deviation 1.26).
Green tea consumption
A total of six studies [14, 36–38, 40, 41], including five case–control studies and one cohort study, were included in the meta-analysis on green tea intake and EC risk. The estimated relative risk of EC for the highest green tea consumption compared with the lowest green tea intake was determined to be 0.78 (95 % CI 0.66–0.92), with no statistically significant heterogeneity observed among the studies (P = 0.16, I 2 = 35.7 %; Fig. 2). When divided by study location, the one study conducted in the USA had a RR of 0.76 (95 % CI 0.48–1.22), whereas the other five studies, which were conducted in Asia, had a RR of 0.79 (95 % CI 0.66–0.94). When divided by study design, the RR of the sole cohort study was determined to be 0.75 (95 % CI 0.43–1.30), whereas the summary RR value for the five case–control studies was 0.79 (95 % CI 0.66–0.94). Sensitivity analyses revealed that the summary RR ranged from 0.75 (95 % CI 0.63–0.88) (excluding the study of Hirose et al. [37]) to 0.81 (95 % CI 0.69–0.96) (excluding the study of Kakuta et al. [38]). Additionally, specific studies were excluded to evaluate the reliability and stability of our conclusions. Excluding the following groups of studies separately did not alter our results: 3 low-quality studies, 2 studies with sample sizes less than one thousand, 4 studies that did not adjust for total energy, 4 studies that did not adjust for diabetes and 3 studies that did not adjust for hormone therapy. Excluding the 4 studies that did not adjust for physical activity provided a non-statistically significant result, but the statistical power was only 6.79 % (Table 3). Dose–response analysis showed that an increase in green tea consumption of one cup per day was statistically significantly associated with a 11 % decrease in the risk of developing EC (RR, 0.89; 95 % CI 0.84–0.94) (Fig. 3).
Black tea consumption
A total of nine studies [10–15, 39, 41, 42] involving four case–control studies and five cohort studies were included in the meta-analysis on black tea intake and EC risk. The summary relative risk estimate of EC for the highest black tea consumption compared with the lowest black tea intake was shown to be 0.99 (95 % CI 0.79–1.23) (Fig. 2), with statistically significant heterogeneity observed among the studies (P = 0.003, I 2 = 64.2 %). Exploration of heterogeneity by subgroup analysis revealed that the observed RRs were similar across the subgroups when they were divided by study location, study design, NOS score and following adjustment for menopausal or hormone therapy (Fig. 4); further, testing for subgroup differences showed no statistical significance among the subgroups. Importantly, only cohort study subgroups and studies of NOS that scored more than 6 stars displayed low heterogeneity (Table 4). Sensitivity analyses revealed that the RR values ranged from 0.92 (95 % CI 0.76–1.12) (excluding the study of Vecchia et al. [15]) to 1.06 (95 % CI 0.87–1.29) (excluding the study of Mccann et al. [39]). Dose–response analysis showed that an increase in black tea consumption of one cup per day did not affect the risk of EC development (RR 0.99; 95 % CI 0.94–1.03) (Fig. 3).
Power analysis and quality of evidence
Power calculations were performed post hoc after all of the studies had been collected using the methodology described by Cafri et al. A power of 84.33 % was determined to detect an RR of 0.78 for the highest green tea consumption compared with the lowest green tea intake and 5.07 % for an RR of 0.99 for the highest black tea consumption compared with the lowest black tea intake. Outcomes in this meta-analysis were evaluated using the GRADE system (Table 5). For green tea, the quality of evidence was determined to be moderate, which was primarily associated with the presence of a dose–response relationship. For black tea, the quality of evidence was very low, which was mainly attributed to serious inconsistency and imprecision between studies.
Publication bias
For green tea intake, there was little evidence of publication bias, as indicated by the Begg rank correlation test (P = 0.707) and the Egger linear regression test (P = 0.763). For black tea intake, there was also no evidence of significant publication bias, as indicated by the Begg rank correlation test (P = 0.99) and the Egger linear regression test (P = 0.127).
Discussion
In this meta-analysis, we found that a higher consumption of green tea was associated with a lower risk of EC. Furthermore, an increase in green tea consumption of one cup per day was statistically significantly associated with an 11 % decrease in the risk of developing EC. In contrast, the consumption of black tea was not shown to have a protective effect against EC.
Heterogeneity is often a concern in meta-analysis; however, we observed little evidence of heterogeneity surrounding the estimate of green tea and EC risk. In contrast, statistically significant heterogeneity was observed among the studies that focused on black tea and EC risk, and subgroup analyses were conducted to further explore this heterogeneity. The observed heterogeneity could partly be explained by study design and NOS score status, stratification by study design revealed 83 % heterogeneity in case–control studies and 0 % heterogeneity in cohort studies. The high heterogeneity observed in the case–control studies may be due in part to the high likelihood of recall and selection biases and to the differential misclassification of exposure, as information on tea consumption was collected after a patient was diagnosed. Furthermore, when a NOS score of six was used to define the quality of studies, 65 % heterogeneity was found in low-quality studies (fewer than six stars), which was in contrast to the 23 % heterogeneity observed in high-quality studies (more than six stars). Together, our results suggest that the quality of primary studies plays an important role in the level of heterogeneity generated in meta-analysis.
A number of potential mechanisms of action have been proposed to account for the cancer-preventive effects of tea and tea constituents. Tea contains large amounts of caffeine and has been shown to induce glutathione-S-transferases, a phase II enzyme that is responsible for the inactivation of dietary and environmental carcinogens [43]. Additionally, and more relevant to the hormonal etiology of endometrial cancer, caffeine has also been shown to affect steroid hormone levels and has been reported to display an inverse association with free estradiol [44] and plasma-free testosterone [45] and a positive association with plasma estrone and sex hormone-binding globulin (SHBG) [44, 45]. In addition, tea contains a number of antioxidants, such as catechins, that could affect carcinogenesis. Indeed, previous studies have demonstrated that epigallocatechin gallate, the major catechin in green tea, can induce apoptosis and cell cycle arrest in human carcinoma cells [46] and can inhibit the estrogen-induced activation of endometrial cells [47]. Tea catechins have also been shown to scavenge relevant reactive oxygen and nitrogen species, such as superoxide, peroxyl radicals, and singlet oxygen [48]. Thus, if the beneficial effect of tea on EC risk comes primarily from the tea catechins, the relative lack of risk reduction associated with black tea consumption can be explained by the much lower level of catechins in black tea than in green tea (up to tenfold difference in catechin contents) [6]. Furthermore, previous studies have also reported a protective effect of tea-gene interaction on EC. For example, Xu et al. [41] found that tea consumption can modify the association of CYP19A1 polymorphisms rs1065779, rs752760, and rs1870050 with endometrial cancer. An inverse association of tea consumption and EC risk was highly evident in individuals with an Asp/Asp genotype of the SHBG Asp327Asn polymorphism, with a particular emphasis observed in premenopausal women [35].
Our study has several strengths compared with the previously reported meta-analyses [9]. To the best of our knowledge, this is the largest systematic review and meta-analysis of observational studies on the intake of green tea/black tea and risk of EC and should thus lead to more reliable results with higher statistical power. In this meta-analysis, the power for RR value of green tea intake and EC incidence was 84.33 %, which indicates that there was sufficient evidence to clarify the inverse association between green tea and EC. Finally, there was little evidence of publication bias for both green/black tea consumption and EC risk studies.
We acknowledge several limitations of our meta-analysis, including the potential misclassification of the type of tea consumed in studies that did not provide aspecific tea type. In those cases, the type of tea was estimated according to local preference. However, we did not estimate the total tea intake and risk of EC because many studies have shown that black and green tea are qualitatively different, with some studies showing that black tea does not have a beneficial effects [49, 50] and other studies finding a positive correlation between black tea consumption and the risk of cancer [51, 52]. In addition, black tea intake has been positively associated with estrogen levels, which has been established as a strong promoter of mammary carcinogenesis [53]. Second, our power for the RR value of black tea intake and EC was only observed to be 5.07 %, which suggests that the estimate is unstable and indicates that more relevant studies are required to further explore this association. Third, as in all observational studies of diet and disease, the possibility of bias and confounding variables should be considered. However, to our knowledge, no clinical randomized controlled trials have been conducted to assess green/black tea consumption and EC risk. Fourth, we recognize that although most of the questionnaires used to generate tea data have been validated and shown to display reasonable reproducibility, a potential misclassification of exposure may be unavoidable. Indeed, most of the included studies measured tea intake at baseline only, rather than updating the information. Thus, changes in tea intake during the follow-up period may result in possible misclassification. Finally, the GRADE quality of evidence for green and black tea and EC risk was moderate and very low, respectively; thus, lowering the confidence in any subsequent recommendations.
In summary, our results suggest that the consumption of green tea, but not black tea, may have a protective effect on EC cancer risk. However, these conclusions should be considered with caution because of the inherent limitations in the described meta-analysis. Large population-based randomized controlled trials and large prospective cohort studies are required to obtain a definitive conclusion and determine the mechanisms underlying this association.
References
Torre LA, Bray F, Siegel RL et al (2015) Global cancer statistics 2012. CA Cancer J Clin 65(2):87–108. doi:10.3322/caac.21262
Svampane L, Strumfa I, Berzina D et al (2014) Epidemiological analysis of hereditary endometrial cancer in a large study population. Arch Gynecol Obstet 289(5):1093–1099. doi:10.1007/s00404-013-3074-7
Brocker KA, Alt CD, Breyer U et al (2014) Endometrial cancer: results of clinical and histopathological staging compared to magnetic resonance imaging using an endorectal surface coil. Arch Gynecol Obstet 289(4):851–858. doi:10.1007/s00404-013-3061-z
Olesen TB, Svahn MF, Faber MT et al (2014) Prevalence of human papillomavirus in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol 134(1):206–215. doi:10.1016/j.ygyno.2014.02.040
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29. doi:10.3322/caac.21254
Balentine DA, Wiseman SA, Bouwens LC (1997) The chemistry of tea flavonoids. Crit Rev Food Sci Nutr 37(8):693–704. doi:10.1080/10408399709527797
Jankun J, Selman SH, Swiercz R et al (1997) Why drinking green tea could prevent cancer. Nature 387(6633):561. doi:10.1038/42381
Lambert JD (2013) Does tea prevent cancer? Evidence from laboratory and human intervention studies. Am J Clin Nutr 98(6 Suppl):1667S–1675S. doi:10.3945/ajcn.113.059352
Tang NP, Li H, Qiu YL et al (2009) Tea consumption and risk of endometrial cancer: a metaanalysis. Am J Obstet Gynecol 201(6):605 e601–605 e608. doi:10.1016/j.ajog.2009.07.030
Weiderpass E, Sandin S, Lof M et al (2014) Endometrial cancer in relation to coffee, tea, and caffeine consumption: a prospective cohort study among middle-aged women in Sweden. Nutr Cancer 66(7):1132–1143. doi:10.1080/01635581.2014.948214
Uccella S, Mariani A, Wang AH et al (2013) Intake of coffee, caffeine and other methylxanthines and risk of Type I vs Type II endometrial cancer. Br J Cancer 109(7):1908–1913. doi:10.1038/bjc.2013.540
Je Y, Hankinson SE, Tworoger SS et al (2011) A prospective cohort study of coffee consumption and risk of endometrial cancer over a 26-year follow-up. Cancer Epidemiol Biomarkers Prevent 20(12):2487–2495. doi:10.1158/1055-9965.epi-11-0766
Giri A, Sturgeon SR, Luisi N et al (2011) Caffeinated coffee, decaffeinated coffee and endometrial cancer risk: a prospective cohort study among US postmenopausal women. Nutrients 3(11):937–950. doi:10.3390/nu3110937
Bandera EV, Williams-King MG, Sima C et al (2010) Coffee and tea consumption and endometrial cancer risk in a population-based study in New Jersey. Cancer Causes Control 21(9):1467–1473. doi:10.1007/s10552-010-9575-9
La Vecchia C, Negri E, Franceschi S et al (1992) Tea consumption and cancer risk. Nutr Cancer 17(1):27–31. doi:10.1080/01635589209514170
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Shafique K, McLoone P, Qureshi K et al (2012) Tea consumption and the risk of overall and grade specific prostate cancer: a large prospective cohort study of Scottish men. Nutr Cancer 64(6):790–797. doi:10.1080/01635581.2012.690063
(2008) A teacupful of medicine? Nat Struct Mol Biol 15(6):537. doi:10.1038/nsmb0608-537
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. doi:10.1007/s10654-010-9491-z
Atkins D, Best D, Briss PA et al (2004) Grading quality of evidence and strength of recommendations. BMJ 328(7454):1490. doi:10.1136/bmj.328.7454.1490
Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30
Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata J 6(1):40
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135(11):1301–1309
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Zhou JG, Tian X, Wang X et al (2015) Treatment on advanced NSCLC: platinum-based chemotherapy plus erlotinib or platinum-based chemotherapy alone? A systematic review and meta-analysis of randomised controlled trials. Med Oncol 32(2):471. doi:10.1007/s12032-014-0471-0
Cafri G, Kromrey JD, Brannick MT (2009) A SAS macro for statistical power calculations in meta-analysis. Behav Res Methods 41(1):35–46. doi:10.3758/BRM.41.1.35
Yun TK, Choi SY (1995) Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiol Biomarkers Prevent 4(4):401–408
Uccella S, Mariani A, Wang A et al (2010) Coffee consumption and the risk of endometrial cancer in postmenopausal women. Gynecol Oncol 116(3):S75–S76
Rossi M, Edefonti V, Parpinel M et al (2013) Proanthocyanidins and other flavonoids in relation to endometrial cancer risk: a case-control study in Italy. Br J Cancer 109(7):1914–1920. doi:10.1038/bjc.2013.447
Takayama S, Monma Y, Tsubota-Utsugi M et al (2013) Food intake and the risk of endometrial endometrioid adenocarcinoma in Japanese women. Nutr Cancer 65(7):954–960. doi:10.1080/01635581.2013.818158
Wang L, Lee IM, Zhang SM et al (2009) Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr 89(3):905–912. doi:10.3945/ajcn.2008.26913
Li WQ, Kuriyama S, Li Q et al (2010) Citrus consumption and cancer incidence: the Ohsaki cohort study. Int J Cancer 127(8):1913–1922. doi:10.1002/ijc.25203
Giri A, Reeves K, Luisi N et al (2011) Coffee, tea consumption and endometrial cancer risk: a prospective cohort study. Am J Epidemiol 173:S233
Xu WH, Zheng W, Cai Q et al (2008) The Asp(327)Asn polymorphism in the sex hormone-binding globulin gene modifies the association of soy food and tea intake with endometrial cancer risk. Nutr Cancer 60(6):736–743. doi:10.1080/01635580802192833
Gao J, Xiang YB, Xu WH et al (2005) Green tea consumption and the risk of endometrial cancer: a population-based case-control study in urban Shanghai. Zhonghualiuxingbingxuezazhi = Zhonghualiuxingbingxuezazhi 26 (5):323–327
Hirose K, Niwa Y, Wakai K et al (2007) Coffee consumption and the risk of endometrial cancer: evidence from a case-control study of female hormone-related cancers in Japan. Cancer Sci 98(3):411–415. doi:10.1111/j.1349-7006.2007.00391.x
Kakuta Y, Nakaya N, Nagase S et al (2009) Case-control study of green tea consumption and the risk of endometrial endometrioid adenocarcinoma. Cancer Causes Control 20(5):617–624. doi:10.1007/s10552-008-9272-0
McCann SE, Yeh M, Rodabaugh K et al (2009) Higher regular coffee and tea consumption is associated with reduced endometrial cancer risk. Int J Cancer 124(7):1650–1653. doi:10.1002/ijc.24125
Shimazu T, Inoue M, Sasazuki S et al (2008) Coffee consumption and risk of endometrial cancer: a prospective study in Japan. Int J Cancer 123(10):2406–2410. doi:10.1002/ijc.23760
Xu WH, Dai Q, Xiang YB et al (2007) Interaction of soy food and tea consumption with CYP19A1 genetic polymorphisms in the development of endometrial cancer. Am J Epidemiol 166(12):1420–1430. doi:10.1093/aje/kwm242
Zheng W, Doyle TJ, Kushi LH et al (1996) Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am J Epidemiol 144(2):175–182
Steinkellner H, Hoelzl C, Uhl M et al (2005) Coffee consumption induces GSTP in plasma and protects lymphocytes against (±)-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide induced DNA-damage: results of controlled human intervention trials. Mutat Res 591(1–2):264–275. doi:10.1016/j.mrfmmm.2005.04.016
London S, Willett W, Longcope C et al (1991) Alcohol and other dietary factors in relation to serum hormone concentrations in women at climacteric. Am J Clin Nutr 53(1):166–171
Ferrini RL, Barrett-Connor E (1996) Caffeine intake and endogenous sex steroid levels in postmenopausal women. The Rancho Bernardo study. Am J Epidemiol 144(7):642–644
Ahmad N, Feyes DK, Nieminen AL et al (1997) Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst 89(24):1881–1886
Laschke MW, Schwender C, Scheuer C et al (2008) Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum Reprod 23(10):2308–2318. doi:10.1093/humrep/den245
Nakagawa T, Yokozawa T (2002) Direct scavenging of nitric oxide and superoxide by green tea. Food Chem Toxicol 40(12):1745–1750
Nie XC, Dong DS, Bai Y et al (2014) Meta-analysis of black tea consumption and breast cancer risk: update 2013. Nutr Cancer 66(6):1009–1014. doi:10.1080/01635581.2014.936947
Tang N, Wu Y, Zhou B et al (2009) Green tea, black tea consumption and risk of lung cancer: a meta-analysis. Lung Cancer 65(3):274–283. doi:10.1016/j.lungcan.2008.12.002
Yu F, Jin Z, Jiang H et al (2014) Tea consumption and the risk of five major cancers: a dose-response meta-analysis of prospective studies. BMC Cancer 14:197. doi:10.1186/1471-2407-14-197
Sun CL, Yuan JM, Koh WP et al (2006) Green tea, black tea and colorectal cancer risk: a meta-analysis of epidemiologic studies. Carcinogenesis 27(7):1301–1309. doi:10.1093/carcin/bgl024
Platet N, Cathiard AM, Gleizes M et al (2004) Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol 51(1):55–67. doi:10.1016/j.critrevonc.2004.02.001
Acknowledgments
This work was supported by Instructional science and technology Program of Changde City (2014ZD11), NSFC (Natural Science Foundation of China) (81360351).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Q. Zhou, H. Li and J.-G. Zhou have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
404_2015_3811_MOESM3_ESM.doc
Supplementary Appendix3: Supplemental characteristics of studies in the meta-analysis of tea intake and EC risk (DOC 38 kb)
Rights and permissions
About this article
Cite this article
Zhou, Q., Li, H., Zhou, JG. et al. Green tea, black tea consumption and risk of endometrial cancer: a systematic review and meta-analysis. Arch Gynecol Obstet 293, 143–155 (2016). https://doi.org/10.1007/s00404-015-3811-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3811-1