Abstract
Purpose
DNA methylation is an important epigenetic modification that is frequently altered in cancer. Recent reports showed that the level of 5-hydroxymethylcytosine (5-hmC) was altered in various types of cancers. The influence of DNA methylation in epithelial ovarian cancer (EOC) is not fully understood. Therefore, the aim of the present study was to investigate factors involved in DNA demethylation in EOC compared with normal ovarian tissues.
Methods
We examined the expression of 5-hmC, 5-mC, and TET2 by immunohistochemistry in 130 cases of EOC and 40 cases of normal ovarian tissues. We assessed the prognostic values of 5-hmC, 5-mC, and TET2 in clinical outcome of EOC.
Results
We discovered a significant decrease in 5-hmC and TET2 expression in EOC compared with normal ovarian tissues. In contrast, there was a significant increase in 5-mC expression in EOC compared with normal ovarian tissues. The expression of 5-hmC, 5-mC, and TET2 correlated with pathologic stage, tumor grading, lymph node metastasis, and vascular thrombosis. Furthermore, decreased level of 5-hmC predicts poor prognosis of EOC patients. The expression of 5-hmC was an independent prognostic factor for overall survival of EOC patients.
Conclusions
The data suggest that loss of 5-hmC is an epigenetic event of EOC, and the expression of 5-hmC could serve as a prognostic factor for EOC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian carcinoma (OC) is the most lethal gynecological malignancy and the fourth most common cause of cancer-related death in women [1]. OC has been predominantly diagnosed in Western countries and its prevalence is gradually increasing in developing countries [2]. Epithelial ovarian cancer (EOC) accounts for the vast majority of adult ovarian malignancies, and is considered to be the most common serous carcinoma [5]. EOC remains one of the most challenging problems in contemporary gynecological oncology worldwide [3] and affects predominantly perimenopausal and postmenopausal women. The frequency of maternal EOC is likely to increase because of the increasing number of women who postpone childbearing [4]. With a 5-year survival rate of only 30 %, EOC is poorly diagnosed; approximately 70 % of cases are diagnosed at advanced stage and early diagnosis is difficult because of vague initial signs and symptoms [6]. Therefore, better knowledge of the molecular pathogenesis of EOC would help contribute both to the identification of new biomarkers useful for its early detection, and to the development of personalized therapies.
Carcinogenesis is a multi-step process that involves certain genomic alterations [8]. These changes not only result from changes in DNA sequences but are also due to epigenetic alterations that result in abnormal gene expression [9–11]. Hypermethylation of tumor suppressors and hypomethylation of oncogenes have been shown to contribute to tumorigenesis [7]. DNA methylation represents a delicate epigenetic modification that may be reversible in mammalian cells. Accumulating evidence suggests a potential link between environmental influences and epigenetic changes. However, the detection of 5-hydroxymethylcytosine (5-hmC) was regarded as a breakthrough in epigenetic research [12, 13].
5-hmC, known as the sixth base of the genome, is related to the process of malignant transformation and is suggested to be an intermediate in active DNA demethylation [14, 15]. 5-hmC is an oxidation product of 5-methylcytosine (5-mC), and formation of 5-hmC from 5-mC automatically lowers the levels of 5-mC at any given nucleotide position or even genome-wide. Therefore, the conversion of 5-mC into 5-hmC could be the first step in a pathway leading towards DNA demethylation [16]. Recent studies demonstrated that the ten-eleven translocation (TET) family of enzymes catalyzes the conversion of 5-mC into 5-hmC [17]. At present, TET mutations influencing DNA methylation have only been found in hematopoietic malignancies, and have not been detected in solid tumors. However, numerous studies have shown that 5-hmC levels are reduced in solid tumor tissues compared with normal tissues, which indicates that TET genes may play a significant role in cellular transformation through regulation of DNA demethylation [18].
As a new epigenetic modification, 5-hmC may be a helpful biomarker to diagnose cancers. To gain a better understand of the roles of TET and 5-hmC in tumors, the biological functions of TET and 5-hmC should be investigated further. Furthermore, there is still a lack of understanding concerning the distribution and importance of 5-hmC and its related enzyme in EOC. Therefore, the purpose of this study was to investigate alterations in 5-mC, 5-hmC, and TET2 expression in EOC compared with healthy ovarian tissues.
Methods
Patients and samples
A total of 130 patients who received surgery for EOC were randomly selected from the medical records of the Department of Pathology at the Second Affiliated Hospital of Harbin Medical University between January 2003 and December 2004. Inclusion criteria included patients who received no treatment prior to surgery. Tumor tissue samples were obtained from all patients at the time of surgery. Forty samples of adjacent normal ovarian tissues were used as controls. Patients were followed up until December 2010.
Immunohistochemistry
Formaldehyde-fixed, paraffin-embedded tissue sections were cut at 4 μm thickness and deparaffinized. Immunostaining was performed with primary antibodies against 5-hmC, 5-mC, and TET2 (Santa Cruz) using a streptavidin–biotin complex method following relevant antigen retrieval techniques according to the standard protocols. Tissues incubated with phosphate-buffered saline instead of primary antibodies were used as negative controls. Specimens were scored according to the intensity of the dye color and the number of positive cells. The intensity of the dye color was graded as 0 (no color), 1 (light yellow), 2 (light brown), or 3 (brown), and the number of positive cells was graded as 0 (<5 %), 1 (5–25 %), 2 (26–50 %), 3 (51–75 %), or 4 (>75 %). The two grades were added together and specimens were assigned to one of four categories: 0–1 (−), 2 (+), 3–4 (++), more than 5 (+++). We designated (− and +) as low expression and (++ and +++) as high expression.
Statistical analysis
We used Pearson’s Chi square test and the Wilcoxon rank-sum test to analyze the association between the expression levels of 5-hmC, 5-mC, TET2, and clinicopathological parameters. Prognostic values of variables were estimated by multivariate Cox proportional hazard ratio models. Survival curves were constructed using the Kaplan–Meier method, and the difference in survival time was evaluated using the log-rank test. The primary endpoint was overall survival (OS). All statistical analyses were performed with GraphPad Prism software version 5.01 and a P value less than 0.05 was considered as statistically significant.
Results
Expression of 5-hmC, 5-mC, and TET2 in EOC and normal ovarian tissues
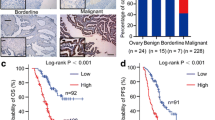
Immunohistochemical analysis of normal ovarian tissues showed a high number of cells positively stained for 5-hmC. We observed similar findings with TET2 expression, although TET2 showed slightly weaker staining. However, in the EOC samples, the numbers of positive 5-hmC and TET2 cells were significantly reduced. We observed an opposite trend with 5-mC expression compared with 5-hmC and TET2. The number of positive 5-mC cells was significantly increased in EOC samples and decreased in normal ovarian tissues (Fig. 1). High expression of 5-hmC, 5-mC, and TET2 in EOC samples was observed in 34 (26.2 %), 90 (69.2 %) and 36 (27.7 %) samples, respectively. Pearson’s Chi square tests revealed that the positive proportion rate was significantly lower for 5-hmC and TET2, and significantly higher for 5-mC in EOC tumor tissues than in normal tissues (P < 0.0001) (Table 1).
Expression of 5-hmC, 5-mC, and TET2 in EOC with clinicopathological factors
We found no statistical significance regarding the expression of 5-hmC, 5-mC, and TET2 in the different histologic types (serous, mucinous, endometrioid, clear cell) (P > 0.05). Reduced 5-hmC expression was more commonly found in the advanced stages of EOC (11 of 73 cases; 15.1 %) than the early stages (23 of 57 cases; 40.4 %) (P = 0.0001). 5-hmC expression was significantly decreased in poorly differentiated EOC tissue (G3) (4 of 33 cases; 12.1 %) compared with well-differentiated EOC tissue (G1) (13 of 29 cases, 44.8 %) (P = 0.013). We observed similar results with TET2 and an opposite trend with 5-mC expression. We also observed positive correlations among the three genes with metastatic lymph node and vascular thrombosis, but no relationship with age, tumor size, CA-125 level, and ascites syndrome (Table 2).
Associations among 5-hmC, 5-mC, and TET2 expression levels in tumor tissues
The interrelationships among the expression levels of 5-hmC, 5-mC, and TET2 are presented in Table 3. Pearson’s Chi square tests revealed that the expression level of 5-mC was significantly inversely associated with those of 5-hmC and TET2 (P < 0.005). Moreover, the expression of 5-hmC was significantly positively associated with TET2 expression (P < 0.005).
Clinical and demographic data of the patients
We reevaluated 2-year data of our center retrospectively between January 2003 and December 2004. The median patient age was 51.6 years. A total of 67.4 % of patients accepted chemotherapy after surgery. The following parameters were observed in this patient group: histologic type (serous 55.4 %, mucinous 23.8 %, endometrioid 9.2 %, clear cell 11.5 %), FIGO Stage (I 14.6 %, II 29.2 %, III 31.5 %, IV 24.6 %), histologic grade (G1 22.3 %, G2 52.3 %, G3 25.4 %), tumor diameter (≤10 cm 54.6 %, >10 cm 45.4 %), metastatic lymph node (positive 63.8 %, negative 36.2 %), vascular thrombosis (positive 49.2 %, negative 50.8 %), serum CA-125 level (≤35 U/ml 30 %, >35 U/ml 70 %) and ascites syndrome (positive 66.2 %, negative 33.8 %).
Prognostic factors for EOC
A total of 130 patients were followed up for up to 96 months, and the end date of the follow-up study was December 2010. The median survival time of patients was 60 months. During the observation period, 55 patients (39.3 %) died.
As displayed in Table 4, lymph node involvement, histology grade, and 5-hmC and TET2 expression were independent significant prognostic factors for OS as shown in the multivariate model including the clinical factors. However, the multivariate analysis revealed that 5-mC was not a significant prognostic factor for OS.
As shown in Fig. 2, low expression of 5-hmC and TET2, and high expression of 5-mC predicted reduced OS of patients with EOC.
Discussion
DNA methylation is an important epigenetic modification and is frequently altered in cancer. Recently, great attention has been paid to the balance between DNA methylation and demethylation in epigenetic modification. Previous studies demonstrated that aberrant DNA methylation in cancer is not only associated with the repression of chromatin related to specific genes, but also with the repression of large chromosomal regions [18]. Conversion of 5-mC to 5-hmC by the TET family enzymes plays significant biological roles in embryonic stem cells, aging and disease. Recent studies found that the level of 5-hmC was altered in various types of cancers. However, these processes in tumor development are still unclear, especially in EOC.
Recently, the discovery of 5-hmC as a novel DNA modification marker in mammalian genomes has generated many questions concerning the role of DNA demethylation in epigenetic regulation. The 5-mC oxidative pathway mediated by the TET proteins may be responsible for activation or repression of gene expression by associating with transcriptional repressors or activation factors [12, 19]. Furthermore, altered 5-hmC was observed in different types of cancers, indicating the 5-hmC pathway may play a significant role in pathogenesis of cancers [17, 20–23]. However, whether the level of 5-hmC is altered in EOC, and whether there is an association between the altered 5-hmC and outcome of patients with EOC has not been clear.
Thus, in this study, we investigated the levels of 5-hmC, TET2, and 5-mC in EOC and normal ovarian tissues. By immunohistochemistry, we discovered significantly lower expressions of 5-hmC and TET2 in EOC compared with normal ovarian tissues. Meanwhile, a higher expression of 5-mC was observed in EOC compared with normal ovarian tissues despite large variations between the samples. Our findings show that increased 5-mC level coincides with decreased 5-hmC and TET2 levels in EOC tissues. These data suggest that the status of DNA methylation may be based on the balance of 5-mC and 5-hmC, and that DNA methylation may be reversible in EOC tumor cells.
Our results were consistent with previous research findings of reduced levels of 5-hmC in other types of cancers [20]. In our study, we demonstrated that low levels of 5-hmC and TET2 and high levels of 5-mC in EOC correlated with pathologic stage, tumor grading, metastatic lymph node and vascular thrombosis. Moreover, the reduced 5-hmC and TET2 levels, and increased 5-mC levels were associated with poor OS. Together these data show that decreased level of 5-hmC predicts poor prognosis of EOC patients. Our data suggest that 5-hmC may act as a prognostic marker for EOC, and that the decreased expression of TET2 is likely one of the mechanisms underlying 5-hmC loss in EOC. Our results also imply that loss of 5-hmC is an epigenetic event in EOC. Interestingly, the distribution and expression of TET2 corresponded to the expression pattern of 5-hmC, which indicates a possible role for TET2 in the loss of 5-hmC expression during carcinogenesis in EOC. This is consistent with the findings by Lian and co-workers, who reported that downregulation of TET2 is one mechanism contributing to the loss of 5-hmC in melanoma [22]. The authors also concluded that increasing TET2 led to re-establishing the 5-hmC level in melanoma cells in vitro and a less aggressive tumor phenotype in an animal model [22].
Our study proves that the loss of 5-hmC in EOC does not result from decreased levels of 5-mC, the substrate of 5-hmC. One study on melanoma suggested that a decrease of 5-hmC leads to an accumulation of 5-mC in melanoma [22]. These findings were further supported by Gambichler et al. [24], who found significantly reduced levels of 5-hmC but no significant reduction of 5-mC in melanoma compared with benign nevi. Loss of 5-hmC has been suggested as a diagnostic biomarker for melanoma [22]. Our findings indicate that loss of 5-hmC may also have a prognostic value in EOC. A marker enabling early diagnosis is of great significance to improve the prognosis of EOC [25]. Further studies are required to investigate whether pre-malignant lesions express low levels of 5-hmC, and if so, whether they show a higher risk of malignant transformation. Our study indicates that loss of 5-hmC is an epigenetic event in EOC, and that the expression of TET2 enzyme corresponds to the expression of its product, 5-hmC.
Taken together, our results revealed that 5-hmC expression is a strong independent predictor for reduced OS, suggesting its prognostic value in EOC. Therefore, we propose that 5-hmC could be a useful marker to predict the prognosis of EOC in clinical practice. Furthermore, 5-hmC may perform an important function in the epigenetic regulation of EOC development.
References
Arikan SK, Kasap B, Yetimalar H, Yildiz A, Sakarya DK, Tatar S (2014) Impact of Prognostic Factors on Survival Rates in Patients with Ovarian Carcinoma. Asian Pac J Cancer Prev 15:6087–6094
Rai Bhavana, Bansal Anshuma, Patel Firuza Darius, Sharma Suresh Chander (2014) Radiotherapy for ovarian cancers-redefining the role. Asian Pac J Cancer Prev 15:4759–4763
Moszynski R, Szubert S, Szpurek D, Michalak S, Krygowska J, Sajdak S (2013) Usefulness of the HE4 biomarker as a second-line test in the assessment of suspicious ovarian tumors. Arch Gynecol Obstet 288(6):1377–1383
He SY, Shen HW, Xu L, Li XL, Yao SZ (2012) Successful management of mucinous ovarian cancer by conservative surgery in week 6 of pregnancy: case report and literature review. Arch Gynecol Obstet 286(4):989–993
Bell DA (2005) Origins and molecular pathology of ovarian cancer. Mod Pathol 18:19–32
Conteduca V, Kopf B, Burgio SL, Bianchi E, Amadori D, De Giorgi U (2014) The emerging role of anti-angiogenic therapy in ovarian cancer (Review). Int J ncol 44:1417–1424
YiChen Wu, Ling ZhiQiang (2014) The role of TET family proteins and 5-hydroxymethylcytosine in human tumors. Histol Histopathol 29:991–997
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Berdasco M, Esteller M (2010) Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell 19:698–711
Jones PA, Liang G (2009) Rethinking how DNA methylation patterns are maintained. Nat Rev Genet 10:805–811
Sharma S, Kelly TK, Jones PA (2010) Epigenetics in cancer. Carcinogenesis 31:27–36
Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A (2009) Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324:930–935
Kriaucionis S, Heintz N (2009) The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324:929–930
Münzel M, Globisch D, Carell T (2011) 5-Hydroxymethylcytosine, the sixth base of the genome. Angew Chem Int Ed Engl 50:6460–6468
Kraus TF, Globisch D, Wagner M, Eigenbrod S, Widmann D, Münzel M, Müller M, Pfaffeneder T, Hackner B, Feiden W, Schüller U, Carell T, Kretzschmar HA (2012) Low values of 5-hydroxymethylcytosine (5hmC), the ‘sixth base’, are associated with anaplasia in human brain tumors. Int J Cancer 131:1577–1590
Pfeifer GP, Kadam S, Jin SG (2013) 5-hydroxymethylcytosine and its potential roles in development and cancer. Epigenetics Chromatin 6:10
Haffner MC, Chaux A, Meeker AK, Esopi DM, Gerber J, Pellakuru LG, Toubaji A, Argani P, Iacobuzio-Donahue C, Nelson WG, Netto GJ, De Marzo AM, Yegnasubramanian S (2011) Global 5-hydroxymethylcytosine content is significantly reduced in tissue stem/progenitor cell compartments and in human cancers. Oncotarget 2:627–637
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358:1148–1159
Iyer LM, Thiliani M, Rao A, Aravind L (2009) Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids. Cell Cycle 8:1698–1710
Yang H, Liu Y, Bai F, Zhang JY, Ma SH, Liu J, Xu ZD, Zhu HG, Ling ZQ, Ye D, Guan KL, Xiong Y (2013) Tumor development is associated with decrease of TET gene expression and 5-methlcytosine hydroxylation. Oncogene 32:663–669
Xu Y, Wu F, Tan L, Kong L, Xiong L, Deng J, Barbera AJ, Zheng L, Zhang H, Huang S, Min J, Nicholson T, Chen T, Xu G, Shi Y, Zhang K, Shi YG (2011) Genome-wide regulation of 5 hmC, 5 mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol Cell 42:451–464
Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, Xu W, Tan L, Hu Y, Zhan Q, Lee CW, Hu D, Lian BQ, Kleffel S, Yang Y, Neiswender J, Khorasani AJ, Fang R, Lezcano C, Duncan LM, Scolyer RA, Thompson JF, Kakavand H, Houvras Y, Zon LI, Mihm MC Jr, Kaiser UB, Schatton T, Woda BA, Murphy GF, Shi YG (2012) Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 150:1135–1146
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of a-ketoglutarate-dependent dioxygenases. Cancer Cell 19:17–30
Gambichler T, Sand M, Skrygan M (2013) Loss of 5- hydroxymethylcytosine and ten-eleven translocation 2 protein expression in malignant melanoma. Melanoma Res 23:218–220
Koyanagi T, Suzuki Y, Saga Y, Machida S, Takei Y, Fujiwara H, Suzuki M, Sato Y (2013) In vivo delivery of siRNA targeting vasohibin-2 decreases tumor angiogenesis and suppresses tumor growth in ovarian cancer. Cancer Sci 104:1705–1710
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
Ethical approval was given by the medical ethics committee of Second Affiliated Hospital of Harbin Medical University with the reference number 2014-yan-037.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Ly., Li, Pl., Wang, Tz. et al. Prognostic values of 5-hmC, 5-mC and TET2 in epithelial ovarian cancer. Arch Gynecol Obstet 292, 891–897 (2015). https://doi.org/10.1007/s00404-015-3704-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3704-3