Abstract
Purpose
The long noncoding RNA HOTAIR has been reported to be a good biomarker for poor prognosis in a variety of human cancers. However, whether HOTAIR could serve as novel biomarker to predict prognosis in cervical cancer or not is unknown. The aim of the present study was to examine the expression of HOTAIR in cervical cancers and to investigate the relationship between this lncRNA expression levels and existing clinicopathological factors and patient survival.
Methods
We examined the expression of HOTAIR in 218 cervical cancer tissues and matched 218 adjacent normal tissues using quantitative real-time RT-PCR and analyzed its correlation with the clinical parameters.
Results
The results showed that HOTAIR expression in cervical cancer tissues was significantly upregulated compared with the matched nontumorous tissues (P < 0.0001). Increased HOTAIR expression was significantly correlated with FIGO stage (P < 0.0001), lymph node metastasis (P < 0.0001), depth of cervical invasion (P < 0.0001), tumor size (P = 0.006) and age (P = 0.020), but not other clinical characteristics. Moreover, cervical cancer patients with HOTAIR higher expression have shown significantly poorer overall survival (P < 0.0001) and disease-free survival (P < 0.0001) than those with lower HOTAIR expression. Univariate (P < 0.0001, HR = 4.566, 95 % CI 2.122–9.825) and multivariate (P = 0.012, HR = 2.863, 95 % CI 1.263–76.490). Cox regression analyses showed that HOTAIR expression served as an independent predictor for overall survival.
Conclusions
our data indicate that high expression of HOTAIR is involved in cervical cancer progression and could be a potential target for diagnosis and gene therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the incidence of cervical cancer has been greatly reduced with the application of pap smear screening, cervical cancer is still the third most commonly diagnosed cancer in women worldwide, with estimated 529,800 new cases diagnosed annually [1]. Moreover, higher incidence rate and mortality rate observed in developing countries versus developed countries [2]. Therefore, it is still urgently for us to find new and effective therapeutic strategies for cervical cancer.
Recent genome sequencing studies have revealed that the human genome is comprised of less than 2 % protein coding genes and more than 90 % of the genome is transcribed as noncoding RNAs [3, 4]. These ncRNAs are classified into two groups depending on the nucleotide size. Those under 200 nucleotides in length are referred to as small ncRNAs, including the microRNAs (approximately 18–25 nucleotides in length) which have been intensively studied [5]. However, the long noncoding RNAs consist of more than 200 nucleotides have relevant limited investigations [6].
HOTAIR (Hox transcript antisense intergenic RNA) is a 2,158 bp lncRNA localized to a boundary in the HOXC gene cluster. Recently, HOTAIR has been identified to overexpression in a variety of cancers such as breast, colon, pancreatic and lung cancer [7–10] and enhanced HOTAIR expression in patients has been correlated with breast and colon cancer metastasis [11–13]. Meanwhile, knockdown of HOTAIR study showed that HOTAIR can inhibit cell invasion, cell proliferation, modulate of cell cycle progression and induce apoptosis, indicating that HOTAIR can play a direct role in the modulation of cancer progression [7–9, 13]. However, little is known about the expression and the impact of HOTAIR in cervical cancer development.
To better understand the role of HOTAIR in cervical cancer development, we investigated the expression of HOTAIR in cervical cancer tissues and analyzed the relationship between this HOTAIR expression levels and existing clinicopathological factors and patient survival.
Materials and methods
Patients and sample collection
Two hundred and eighteen cervical cancer tumors and 218 matched adjacent normal tissues were obtained from Sun Yat-sen University Cancer Center (SYSUCC) (Guangzhou, China) between January 2005 and October 2009. All patients recruited in this study were not subjected to preoperative radiotherapy and/or chemotherapy and were diagnosed as infiltrating carcinoma by pathology. The tumor stage was classified by two experienced gynecological oncologists according to the International Federation of Gynecology and Obstetrics (FIGO) staging system for cervical cancer. Clinical and pathological variables analyzed are shown in Table 1. All patients were regularly followed up, with a mean observation period of 42 months (range 2–55 months). Tissue specimens were immediately stored at 4 °C for 24 h in RNA Later (Ambion Inc.), then at −80 °C liquid nitrogen until the extraction of total RNA. Total RNA from fresh cervical cancer tissues was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The study was approved by the Medical Ethics Committee of Sun Yat-sen University Cancer Center.
Quantitative real-time reverse transcriptase PCR
The quality and concentration of RNA were determined using a Nanodrop 1000 (Thermo, Wilmington, DE, USA). Then, 1 µg total RNA reversely transcribed from each sample to synthesis cDNA by the RT reagent Kit (Promega, USA, A3500) according to the manufacturer’s instructions with minor modification. Real-time PCR was performed to determine the relative expression level of target genes by the SYBR Green qRT-PCR kit (Promega, USA, A6001) on the CFX96 Real-Time PCR Detection System (Bio-Rad). The level of HOTAIR expression in each sample was normalized to the respective GAPDH expression level. The primers used were as follows: HOTAIR sense, 5′-CAGTGGGGAACTCTGACTCG-3′ and antisense, 5′-GTGCCTG GTGCTCTCTTACC-3′; GAPDH sense, 5′-CTCCTCCTGTTCGACAGTCAGC-3′ and antisense, 5′-CCCAATACGACCAAATCCGTT-3′. The amplification profile was 95 °C for 5 min, followed by 42 cycles of denaturation at 95 °C for 15 s, annealing and extension at 60 °C for 60 s. The specificity of each PCR was confirmed by melting curve analyses. The comparative Ct method (ΔΔCt) was used for quantification of the gene expression. The relative expression amount of HOTAIR to GAPDH was calculated using the equation 2−ΔΔCt, where ΔCt = Ct HOTAIR−Ct GAPDH. The gene expression levels of HOTAIR in tumor were compared with normal tissues. To minimize experimental variability, each sample was analyzed in triplicate and the mean expression level was calculated.
Statistical analysis
Statistical analysis was carried out with SPSS software package (IBM, standard version 18.0). The relationship between HOTAIR expression and clinicopathological characteristics was assessed using Pearson’s χ 2 test. Survival curves were estimated by the Kaplan–Meier method. The log-rank test was used to estimate the statistical differences between survival curves. Cox proportional hazards analysis was performed to calculate the hazard ratio (HR) and the 95 % confidence interval (CI) to evaluate the association between HOTAIR expression and survival. Multivariate survival analysis was carried out for all of the parameters that were significant in the univariate analysis using the Cox regression model. A two-sided P value of <0.05 was considered statistically significant.
Results
HOTAIR was overexpressed in cervical cancer
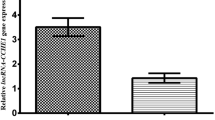
The HOTAIR expression levels were detected in 218 cervical cancer samples and adjacent, histologically normal tissues by qRT-PCR. HOTAIR expression was significantly upregulated in cancerous tissues compared with its own surrounding tissues (2.383 ± 0.152, P < 0.0001) (Fig. 1a). Furthermore, the results revealed a significant association between HOTAIR upregulation and advanced FIGO stage (IB1 vs. IB2-IIA vs. IIB, P < 0.001) (Fig. 1b) and lymph node metastasis (metastasis vs. not metastasis, P = 0.012; number of positive lymph node = 0 vs. number of positive lymph node = 1 vs. number of positive lymph node ≥2, P = 0.018) (Fig. 1c, d).
HOTAIR expression levels were detected in 218 cervical cancer samples and adjacent normal tissues by qRT-PCR. a HOTAIR expression was significantly upregulated in cancerous tissues compared with corresponding normal tissues. b HOTAIR expression was significantly higher in tumors of higher p FIGO stage than that in tumors of lower FIGO stage. The expression of HOTAIR increased along with the number of lymph node metastasis, c metastasis (M) vs. not metastasis (NM); d number of positive lymph node = 0 vs. number of positive lymph node = 1 vs. number of positive lymph node ≥2). Expression levels are normalized to GAPDH. The relation expression of HOTAIR was compared with the median expression value of HOTAIR of normal tissues. Error bars represent SE
Correlations between the expression of HOTAIR and the clinicopathological factors in cervical cancer
To identify the clinical relevance of HOTAIR expression in cervical cancer, correlation between HOTAIR expression and clinicopathological parameters such as age, histology, FIGO stage, tumor differentiation, SCC-Ag, tumor size, depth of cervical invasion, uterine corpus invasion and lymph node metastasis was examined. Of the 218 human cervical cancer tissues were further classified into the high-HOTAIR group (n = 109) and low-HOTAIR group (n = 109) using the median expression value of HOTAIR as the cutoff point. The results showed that increased HOTAIR expression was significantly correlated with FIGO stage (P < 0.0001), lymph node metastasis (P < 0.0001), depth of cervical invasion (P < 0.0001), tumor size (P = 0.006) and age (P = 0.020), but not other clinical characteristics (Table 1).
Association between HOTAIR expression and prognosis of cervical cancer patients
Overall survival curves and disease-free survival curves in different FIGO stage group are shown in Fig. 2a, b. As was expected, patients with advanced FIGO stage have shown significantly poorer overall survival (P < 0.0001) and disease-free survival (P < 0.0001). Overall survival curves and disease-free survival curves in high-HOTAIR group and low-HOTAIR group are shown in Fig. 2c, d. Patients with high HOTAIR expression have shown significantly poorer overall survival (P < 0.0001) and disease-free survival (P < 0.0001) than those with low HOTAIR expression.
Kaplan–Meier survival curve analysis shows that FIGO stage was significantly associated with a poor overall survival and b disease-free survival in 218 cervical cancer cases. Patients with higher expression of HOTAIR showed decreased c overall survival and d disease-free survival compared with patients with lower expression of HOTAIR. P value was calculated by Log-rank test
Univariate and multivariate analyses show HOTAIR expression is an independent predictor for overall survival
As shown in Table 2, univariate analysis identified four prognostic factors: FIGO stage (IB2-IIB vs.IB1), depth of cervical invasion (≥2/3 vs. <2/3), lymph node (positive vs. negative) and HOTAIR expression (high vs. low). The other clinicopathological characteristics, such as age, histology, differentiation, SCC-Ag, tumor size and uterine corpus invasion, were not statistically significant prognosis factors. Parameters that were significantly related to survival in univariate analysis were entered into the multivariate analysis. When it comes to multivariate, Cox regression model revealed that only FIGO stage (P < 0.0001, HR = 1.994, 95 % CI 1.359–2.927), lymph node metastasis (P = 0.005, HR = 2.636, 95 % CI 1.348–5.156) and HOTAIR expression level (P = 0.012, HR = 2.863, 95 % CI 1.263–6.490) served as independent prognostic factors for poor overall survival.
Receiver operating characteristic (ROC) curve analysis
ROC curve analysis showed that the HOTAIR expression is a good candidate to discriminate tumor tissues from nontumorous tissues (sensitivity: 60.6 %, specificity: 87.2 %) and the presence or absence of lymph node metastasis (sensitivity: 85.1 %, specificity: 64.9 %). The areas under the ROC curve (AUC) are 0.803 (95 % CI 0.762–0.839, P < 0.0001) and 0.802 (95 % CI 0.742–0.852, P < 0.0001), respectively (Fig. 3).
Receiver operating characteristic curve (ROC) analysis was employed to determine whether HOTAIR is really good candidates to discriminate (a) tumor tissues from nontumorous tissues and (b) the presence or absence of lymph node metastasis. The areas under the ROC curve (AUC) are 0.803 (95 % CI 0.762–0.839, P < 0.0001) and 0.802 (95 % CI 0.742–0.852, P < 0.0001), respectively
Discussion
LncRNAs have been recently implicated as having oncogenic [14] and tumor suppressor roles [15]. In this study, we found enhanced expression of HOTAIR in cervical cancer tissues compared with adjacent normal tissues. We showed for the first time that HOTAIR was frequently up-regulated in cervical cancer. These findings are consistent with the recent study showing that upregulation of HOTAIR in a variety of cancers such as breast, colon, pancreatic and lung cancer. Our results also revealed that the high level expression of HOTAIR was associated with lymph node metastasis. These findings are also consistent with a study showing that upregulation of HOTAIR is correlated with lymph node metastasis of patients with gastric cancer [16]. Moreover, the current study showed that HOTAIR knockdown can inhibit the invasion and metastasis of NSCLC in vitro and in vivo [17]. Therefore, we hypothesis HOTAIR may involved in the progression of cervical cancer through promoting the invasion metastasis.
Although evidence of carcinogenicity of HOTAIR in humans is strong, the molecular mechanism by which tumor development and metastasis are promoted is not fully understood. HOTAIR resided in the HOXC locus, but repressed transcription in the more distal HOXD locus in foreskin fibroblasts. It has been revealed that HOTAIR interacted with the Polycomb Repressive Complex 2 (PRC2) complex, a methyl-transferase that trimethylates histone H3 at lysine 27 (K27) to repress transcription and up to 20–40 % known lncRNAs are associated with it [18–20] and was required for PRC2-dependent histone H3 lysine 27 trimethylation and gene silencing. Alteration of HOTAIR expression in cancer cells results in both enhanced and suppressed expression of genes, and a subset of genes repressed by HOTAIR was also coregulated by PRC2 [21, 22], suggesting that HOTAIR-mediated suppression of genes in cancer cells is PRC2 dependent.
In this study, GAPDH was selected to be the house-keeping gene. The expression data of HOTAIR were based on GAPDH. Although some articles reported that GAPDH has been shown to be upregulated in many cancers and downregulated by chemotherapic drugs [23, 24], we have confirmed that GAPDH is not itself differentially repressed in cervical cancer tissue in our previous study.
In conclusion, in our study, for the first time, we reported that HOTAIR was frequently upregulated in cervical cancer tissues, and as an independent prognostic factor in patients with cervical cancer. This new findings indicate that HOTAIR may be used as a potential target for diagnosis and gene therapy of cervical cancer. However, the precise molecular mechanisms of HOTAIR that involved in cervical cancer need to be further studied.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Schiffman M, Castle PE, Jeronimo J et al (2007) Human papillomavirus and cervical cancer. Lancet 370:890–907
The ENCODE Project Consortium (2004) The ENCODE (ENCyclopedia of DNA Elements) project. Science 306:636–640
The ENCODE Project Consortium (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447:799–816
Filipowicz W, Bhattacharyya SN, Sonenberg N (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers insight? Nat Rev Genet 9(2):102–114
Sotillo E, Thomas-Tikhonenko A (2011) The long reach of noncoding RNAs. Nat Genet 43(7):616–617
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM et al (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464:1071–1076
Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S et al (2011) Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res 71:6320–6326
Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J et al (2013) HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 32:1616–1625
Nakagawa T, Endo H, Yokoyama M, Abe J, Tamai K et al (2013) Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun 436:319–324
Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA et al (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129:1311–1323
Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F et al (2010) Long noncoding RNA as modular scaffold of histone modification complexes. Science 329:689–693
Yang Z, Zhou L, Wu LM, Lai MC, Xie HY, Zhang F et al (2011) Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol 18:1243–1250
Ge X, Chen Y, Liao X, Liu D, Li F, Ruan H, Jia W (2013) Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of poor prognosis in patients with colorectal cancer. Med Oncol 30(2):588
Han L, Kong R, Yin DD, Zhang EB, Xu TP, De W, Shu YQ (2013) Low expression of long noncoding RNA GAS6-AS1 predicts a poor prognosis in patients with NSCLC. Med Oncol 30(4):694
Hajjari M, Behmanesh M, Sadeghizadeh M, Zeinoddini M (2013) Up-regulation of HOTAIR long non-coding RNA in human gastric adenocarcinoma tissues. Med Oncol 30:670
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX, De W (2013) The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer 13(1):464
Saga Y, Mizukami H, Suzuki M, Kohno T, Urabe M, Ozawa K, Sato I (2002) Overexpression of PTEN increases sensitivity to SN-38, an active metabolite of the topoisomerase I inhibitor irinotecan, in ovarian cancer cells. Clin Cancer Res 8(5):1248–1252
Hu YC, Lam KY, Law S, Wong J, Srivastava G (2001) Identification of differentially expressed genes in esophageal squamous cell carcinoma (ESCC) by cDNA expression array: overexpression of Fra-1, Neogenin, Id-1, and CDC25B genes in ESCC. Clin Cancer Res 7(8):2213–2221
Aguilo F, Zhou MM, Walsh MJ (2011) Long noncoding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a expression. Cancer Res 71(16):5365–5369
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ et al (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464:1071–1076
Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T et al (2011) Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res 71:6320–6326
Hansen CN, Ketabi Z, Rosenstierne MW, Palle C, Boesen HC, Norrild B (2009) Expression of CPEB, GAPDH and U6snRNA in cervical and ovarian tissue during cancer development. APMIS 117(1):53–59
Harada N, Yasunaga R, Higashimura Y, Yamaji R, Fujimoto K, Moss J et al (2007) Glyceraldehyde-3-phosphate dehydrogenase enhances transcriptional activity of androgen receptor in prostate cancer cells. J Biol Chem 282(31):22651–22661
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, L., Liao, LM., Liu, AW. et al. Overexpression of long noncoding RNA HOTAIR predicts a poor prognosis in patients with cervical cancer. Arch Gynecol Obstet 290, 717–723 (2014). https://doi.org/10.1007/s00404-014-3236-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3236-2