Abstract
Objective
To present physiologic intraoperative data and immediate postoperative outcomes of patients diagnosed with epithelial ovarian cancer submitted to cytoreductive surgery and hyperthermic peritoneal intraoperative chemotherapy (HIPEC) with a closed-circuit, turbulent-flow system.
Materials and methods
A closed-circuit system with CO2 turbulent flow was used for paclitaxel HIPEC during 60 min for patients diagnosed with stage II or higher and recurrent epithelial ovarian cancer. Perioperative hemodynamic and metabolic statuses were followed, as well as physiologic recovery during the first 12 postoperative hours. A non-parametric statistical analysis was performed.
Results
At the end of the hyperthermia phase, temperature was 37.7 ± 0.6 °C, heart rate 88 ± 19 bpm, cardiac index 2.8 ± 0.5 L min−1 m−2, stroke volume variation 14.6 ± 3.6 % and extravascular lung water 8.7 ± 1.9 mL kg−1. No hyperdynamic status was recorded. The length of stay in the ICU was 2½ days, and 12.7 ± 7 days in hospital. Average postoperative intubation time was 11.7 ± 17.4 h. At the ICU admission time, glucose, lactic acid and hemoglobin were the only values out of range, but close to normal. SOFA median was 3 at admission and 0 the following day.
Conclusion
A turbulent-flow, closed-circuit use for hyperthermic peritoneal intraoperative chemotherapy resulted in no hyperdynamic response or coagulopathy, had good tolerance and promoted early physiologic recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When epithelial ovarian cancer (EOC) is diagnosed, it is already disseminated across the peritoneal surface in 70–80 % of the patients, 50 % has lymphatic infiltration and 13 % has metastatic disease [1]. Front-line treatment includes cytoreductive surgery (CRS) and adjuvant chemotherapy, usually with carboplatin. The amount of residual tumoral tissue after CRS is key to determine overall survival (OS) of EOC patients. Intraoperative hyperthermia might be synergistic with chemotherapy in the peritoneal cavity [2], enhancing tumor cell killing capacity. Based on the uncontrolled series [3], the use of hyperthermic intraperitoneal chemotherapy (HIPEC) makes sense for advanced and recurrent EOC for the time being, but definitive data from randomized clinical trials [4] remain to be seen. The classic open-abdomen coliseum technique for HIPEC is currently being substituted in most centers for closed-circuit techniques. The latter has various theoretical advantages: increased ability to quickly achieve and maintain hyperthermia, increased pressure of the chemotherapeutic-containing solution upon the peritoneal surfaces, more stable patient temperature and physiologic response, minimal heat loss and minimized exposure of the operating room staff to chemotherapy drugs [5, 6]. We report our HIPEC pilot series testing security and efficiency of a newly designed closed-circuit turbulent-flow device and compare our early outcomes with other available series. Once the performance of the device is checked by this pilot series, a randomized trial focused on long-term oncologic outcomes will be started in the near future (EUDRA-CT 2011-006319-69).
Materials and methods
Women diagnosed with EOC class II and higher as well as recurrent EOC were submitted to CRS and HIPEC at the Hospital General Universitario de Ciudad Real from November 15 2011 to January 31 2013, fulfilling a pilot program previous to a randomized clinical trial to be started latter on, during 2013 (EUDRA-CT 2011-006319-69). This pilot program was approved by the ethics committee. All patients gave written consent before they were included. The standard surgical procedure included a cytoreductive phase: hysterectomy and bilateral oophorectomy (except patients who already had a previous incomplete surgery and patients suffering a relapsed episode of their ovarian cancer disease), lymphadenectomy of the pelvic and paraaortic areas and resection of peritoneal implants. Once the cytoreductive surgery was completed, the HIPEC phase took place using paclitaxel [7, 8] (175 mg m−2) at 42–43 °C during 60 min. Paclitaxel molecule has a safe and effective profile for HIPEC use: high molecular weight (853.9 Dalton), responsible for the low systemic absorption rate and high peritoneal concentration [9]. Bowel and liver resection, in case indicated, was done before the HIPEC phase but the intestinal anastomoses were completed afterwards to preserve it from any possible deleterious effect of HIPEC.
All patients received a balanced general anesthetic. A thoracic epidural placed in awake patients at T7–T8 level was considered for perioperative management in some patients per anesthesiologist criteria. Only three anesthesiologists were involved in this series. All patients were monitored with a Pulse Contour Cardiac Output PiCCO (Pulsion® Medical Systems AG, Munich, Germany) device inserted in the radial artery [10]. Blood temperature was monitored by the in-line PiCCO probe (tip situated in the superior cava) in addition to an esophageal probe (GE Healthcare Finland Oy, Helsinki, Finland). A norepinephrine infusion was started at low doses with a damage-control intention to ameliorate the rate of IV fluids that were infused whenever such rate was deemed excessive (>1/2 L h−1) and systemic vascular resistance (SVR) was not above normal. Dobutamine was used in case Cardiac Index (CI) was lower than 2.0 L min-1 m-2 and unresponsive to fluid resuscitation. Signs of under-resuscitation for our goal-directed fluid management [11] were Stroke Volume Variation SVV >13 % as main criteria and secondly Global end-diastolic volume (GEDVI) <650 mL m−2. Gloves, goggles and a respiratory mask filter (FFP3D type, DACH™ Schutzbekleidung GmbH & Co. KG, Bietigheim, Germany) were used by operating room personnel during the HIPEC phase. The patients were transported to the surgical ICU and intubated after the surgery was completed. Complications noticed during the ICU stay or latter on in the ward were classified using a previously validated system [Criteria NCICT for adverse events (CTCAE) version 4.0. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-014_QuickReference_8.5x11.pdf. Accessed March, 2013]. This classification is disclosed as follows. Grade 1, Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated. Grade 2, Moderate; minimal, local or noninvasive intervention indicated; limiting age-appropriate Activities of Daily Living. Grade 3, Severe or medically significant but not immediately life threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self care Activities of Daily Living. Grade 4, Life-threatening consequences; urgent intervention indicated. The patients were scheduled to receive 6 follow-up cycles of IV carboplatin (AUC 6) and paclitaxel at 175 mg m−2 postoperatively.
We used for our patients a newly designed closed circuit, the Combat PRS™ (Peritoneum Recirculation System, Galmaz Biotech, Madrid, Spain) (Figs. 1 and 2). This model combines three parallel recirculation circuits: two for fluids and one for CO2. Pressures are maintained within a 12–15 mm Hg range, flow rate at 2,000–2,400 mL min−1 and temperature 41–43 °C. CO2 recirculation generates an intra-abdominal turbulent flow that facilitates a homogeneous distribution of the perfusion solution across the peritoneal surfaces. The entrance multiperforated tube was placed under the left diaphragm, running between the descending colon and the abdominal wall, crossing the pelvis and up to the right diaphragm between the ascending colon and the abdominal wall. On the contrary, the exit drain was placed in the pelvis, running up to the right diaphragm, towards the left side and down to the pelvis. CO2 was inflated through a tube positioned behind the mesenteric root to elevate and separate the intestinal loops. CO2 was passively exited at the abdominal cupola with a corrugate tube, closing the system with no leaks. Fluid temperature was tested at the entrance and exit tubes. The usual volume necessary to fill-up the circuit was 4 ± 0.5 L. The solution contained glucose 75.7 mmol L−.1, sodium 132 mmol L−1, calcium 1.75 mmol L−1, Magnesium 0.25 mmol L−1, chloride 101 mmol L−1, bicarbonate 25 mmol L−1, sorbitol 10 mmol L−1; osmolarity 345 mOsm L−1; and pH 7.4. The skin and subcutaneous tissue were closed with a running suture for the HIPEC phase. A circuit and peritoneal cavity lavage was done after HIPEC and the in–out volume difference was calculated to figure out residual volume, transperitoneal fluid absorption and circuit spillage to estimate room staff contamination risk. Bowel anastomoses were completed after the HIPEC phase.
Literature searches in MEDLINE, LILACS, Google Scholar and Cochrane Library Databases were done using “HIPEC” and “postoperative outcomes”, “HIPEC” and “complications”, “HIPEC” and “hemodynamics”. We obtained 81 results. Twenty were repeated. After abstract review, 13 articles were ruled out due to unrelated contents. After full review of the articles, 21 were discarded due to non-usable information. We included 27 articles in our review.
Preoperative, intraoperative and postoperative data (hemodynamic and laboratory outcomes and clinical complications) were collected prospectively and entered into a computerized database. The principal variables were time to extubation and SOFA (Sequential Organ Failure Assessment) score. Qualitative outcomes were presented as median and 25–75th ‰ and quantitative outcomes as mean and standard deviation. The Wilcoxon signed rank sum test (coupled comparison) or Friedman test (serial comparison) was used to compare paired variables. Spearman’s rho was used to establish correlations of temperature, time to extubation, Simplified Acute Physiology Score (SAPS), Acute Physiology and Chronic Health Evaluation (APACHE) II and SOFA. Significance was accepted with p values 0.05 or lower.
Results
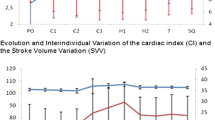
Fifteen patients were submitted to cytoreductive surgery and HIPEC with a closed-circuit, turbulent-flow system. Age, past medical history, histological features and surgery extent are depicted in Table 1. An intraoperative norepinephrine infusion was used in 7 cases. Dobutamine was never used. The average total procedure time (anesthetic and surgical time altogether) was 492 ± 54 min. Total IV fluid infusion rate was 10 mL kg−1 h−1. We used a colloid–crystalloid ratio of 1:4. Intraoperative urine output was 113 ± 50 mL h−1. A low thoracic epidural was placed in 9 patients. Temperature, IV fluids, blood products use, metabolic status and hemodynamic changes related to the HIPEC phase are shown in Table 2. Blood temperature was correlated with GEDVI (ρ = 0.69; p = 0.004) by the end of the HIPEC phase. The necessary volume to fill-up the circuit was 4.2 ± 0.4 L. The in–out volume difference ranged 465–1,220 ml (Fig. 3).
The physiological status at the arrival to the ICU appears in Table 3. The length of stay (LOS) in the ICU was consistently 60 ± 5 h (2½ days) and 12.7 ± 7 days in hospital. APACHE II at ICU admission correlated with SVV at the end of HIPEC (ρ = 0.7; p = 0.003). Median intubation time was 6 h. Only three cases remained intubated more than 12 h and only one patient more than 24 h (actually, 72 h). The changes in the physiologic status of our population and SOFA score 12 h after admission (day 2) are shown in Table 4; the score on day 2 correlated with low prothrombin time by the end of the HIPEC phase (ρ = −0.62; p = 0.04). Urine output was 107 ± 44 mL h−1 during those 12 h, down to 86 ± 32 mL h−1 the following day (p = 0.01). The first day volume balance in the ICU was 2,166 ± 956 mL, down to 936 ± 1,745 mL (p = 0.03) the next day.
We had 18 grade 1 complications in 12 patients (morbidity 80 %), which included nausea and vomiting (n = 1), metabolic acidosis (n = 4), pleural effusion (n = 1), neutropenia (n = 4), low platetet count (n = 2), arterial ischemia (n = 1), ileus (n = 2), delirium (n = 1) and hematuria (n = 1); 15 grade 2 complications in 7 patients (morbidity 46.7 %), which included reintubation (n = 1), soft tissue collection (n = 1), deep vein thrombosis (n = 1), ascitic leak (n = 1), pneumonia (n = 3), vesicle leak (n = 1), packed red blood cells (PRBCs) transfusion (n = 3), fresh frozen plasma (FFP) transfusion (n = 1) and platelets transfusion (n = 1); two grade 3 complications (morbidity 13.3 %), which included eventration and soft tissue collection. One patient died suddenly on the 11th postoperative day (mortality 6.6 %) while she was recovering from respiratory complications. Thirteen out of 15 were discharged home by postoperative day 28, and 14 out of 15 by day 90 (one patient deceased).
Discussion
Our patient population age was older than other reported series but the co-morbidities prevalence was standard [12]. Our total IV fluid infusion rate of 10 mL kg−1 h−1 was above the standard requirement for major abdominal surgery (6–8 mL kg−1 h−1), but it was not as high as the usual rate described for cytoreductive surgery (12 mL kg−1 h−1) [13].
Our group has studied the intra-abdominal fluid flow pattern in an experimental closed-circuit model in piglets [Sánchez García S, Villarejo Campos P, Padilla Valverde D et al. Experimental intraabdominal chemotherapy closed-circuit model with CO2 recirculation. 29th Congreso Nacional de Cirugía, Madrid, November 15th 2012 (abstract), Spanish]. The fluid infused contained methylene blue. The peritoneal cavity was examined afterwards and a consistent pattern staining certain areas of the surface and preserving others was noticed. This problem has been pointed out before [14]. We concluded that such systems did not guarantee a homogeneous distribution of the chemotherapeutic agent across the peritoneal surfaces. Such circumstance could be important not only as far as tumor cell killing capacity, which is out of the scope of the present article, but could also potentially influence early outcomes. This closed system did not induce a temperature raise higher than previously described by the end of the HIPEC phase (top average 37.7 °C) [15]. Heart rate and SVV increased during the HIPEC phase revealing the vasodilatation and relative volume deficit associated with the heat increase [16]. It was not accompanied by a decrease in preload (GEDVI) probably due to the rise of intra-abdominal pressure, a mechanism already described [17]. CPI was rocky stable, but the resulting CI was slightly decreased. SVRI had a non-significant tendency to decrease. Therefore, we did not encounter the hyperdynamic situation generally described [18], but we have not been the only ones to find this moderate behavior [19]. There was an ELWI elevation before, during and after the HIPEC phase. The possible relation of these data to the low plasma protein content [20] once the ascites is drained has been pointed out [21]. In our case, the preoperative total protein was within a normal range, but it dropped down during the procedure (2.7 g dL−1 drop; p = 0.03). The low oncotic pressure did not create a lung permeability problem, as PVPI was always preserved normal. The metabolic end-organ demand that parallels temperature was responsible for the moderate lactic acidosis encountered.
There has been some debate about the risk–benefit of using a perioperative LTE [22]. We, as others [23], did not detect a difference in hemodynamic parameters or IVF management during the procedure (total amount of IV fluid p = 0.6; GEDVI p = 0.4; SVV p = 0.9) associated with the presence or not of an LTE. There were two cases with a postoperative platelet count below 100,000 but above 50,000, which should not represent a problem at the time of removing the catheter. We conclude that LTE can be safely but judiciously used for perioperative management.
Renal function was well preserved without difficulty, just as already known [24]. No hyperglycemia, hyponatremia [25] or hyperkalemia was noted in our series. Laboratory analysis revealed a moderate intraoperative change in coagulation parameters: decrease of prothrombin activity (PT) to 76 %, no change in activated partial thromboplastin time and a reduced number of thrombocytes compared to preoperative level, but still within normal limits. These are minor changes compared to findings communicated by other series [26].
The SOFA score was already low on admission at the ICU, and most laboratory values were within normal range except, lactic acid, glucose and hemoglobin, which were close to normal and basically acceptable as postoperative values. Twelve hours latter median SOFA was 0.
Table 5 summarizes early outcomes reported in previous series. Despite the fact that most authors use similar classification systems, there are obvious disparities about the particular criteria applied. Inter-studies variation is enormous and comparisons are problematic at the least. Therefore, conclusions about the effectiveness of our turbulent-flow device cannot be based on direct comparison between different systems. Our immediate postoperative outcome was reassuring per se. During the initial hours in the ICU, the evolution was very favorable. We did not find some of the problems previously attributed to HIPEC, as hyperdynamic status or coagulopathy.
References
Helm CW (2012) Current status and future directions of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of ovarian cancer. Surg Oncol Clin N Am 21:645–663
Chang E, Alexander HR, Libutti SK, Hurst R, Zhai S, Fiff WD, Bartlett DL (2001) Laparoscopic continuous hyperthermic peritoneal perfusion. J Am Coll Surg 193:225–229
Kusamura S, Younan R, Baratti D, Costanzo P, Favaro M, Gavazzi C, Deraco M (2006) Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer 106:1144–1153
Chan DL, Morris DL, Rao A, Chua TC (2012) Intraperitoneal chemotherapy in ovarian cancer, a review of tolerance and efficacy. Cancer Manag Res 4:413–422
Gesson-Paute A, Ferron G, Thomas F, de Lara EC, Chatelut E, Querleu D (2008) Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted heated intraoperative intraperitoneal chemotherapy (HIPEC): an experimental study. Ann Surg Oncol 15:339–344
Tsiftsis D, de Bree E, Romanos J, Petrou A, Sanidas E, Askoxylakis J, Zervos K, Michaloudis D (1999) Peritoneal expansion by artificially produced ascites during perfusion chemotherapy. Arch Surg 134:545–549
Kim JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, Lee KH, Lee SH, Kim SJ (2010) Consolidation hyperthermic intraperitoneal chemotherapy using paclitaxel in patients with epithelial ovarian cancer. J Surg Oncol 101:149–155
Panici PB, Palaia I, Graziano M, Bellati F, Manci N, Angioli RI (2010) Intraperitoneal paclitaxel as consolidation treatment in ovarian cancer patients: a case control study. Oncology 78:20–25
de Bree E, Rosing H, Filis D, Romanos J, Melisssourgaki M, Daskalakis M, Pilatou M, Sanidas E, Taflampas P, Kalbakis K, Beijnen JH, Tsiftsis DD (2008) Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: a clinical and pharmacokinetic study. Ann Surg Oncol 15:1183–1192
Hofer CK, Senn A, Weibel L, Zollinger A (2008) Assessment of stroke volume variation for prediction of fluid responsiveness using the modified FloTrac and PiCCOplus system. Crit Care 12:R82
Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM (2012) Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg 114:640–651
Chia VM, O’Malley CD, Danese MD, Lindquist KJ, Gleeson ML, Kelsh MA, Griffiths RA (2013) Prevalence and Incidence of comorbidities in elderly women with ovarian cancer. Gynecol Oncol 129(2):346–352
Raspe C, Piso P, Wiesenack C, Bucher M (2012) Anesthetic management in patients undergoing hyperthermic chemotherapy. Curr Opin Anaesthesiol 25:348–355
Benoit L, Cheynel N, Ortega-Deballon P, Di Giacomo G, Chauffert B, Rat P (2008) Closed hyperthermic intraperitoneal chemotherapy with open abdomen: a novel technique to reduce exposure of the surgical team to chemotherapy drugs. Ann Surg Oncol 15:542–546
Esquivel J, Sticca R, Sugarbaker P, Society of Surgical Oncology et al (2007) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Ann Surg Oncol 14:128–133
Cafiero T, Di Iorio C, Di Minno RM, Sivolella G, Confuorto G (2006) Non-invasive cardiac monitoring by aortic blood flow determination in patients undergoing hyperthermic intraperitoneal intraoperative chemotherapy. Minerva Anestesiol 72:207–215
Bickel A, Arzomanov T, Ivry S, Zveibl F, Eitan A (2004) Reversal of adverse hemodynamic effects of pneumoperitoneum by pressure equilibration. Arch Surg 139:1320–1325
Esquivel J, Angulo F, Bland RK, Stephens AD, Sugarbaker PH (2000) Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open ‘‘coliseum technique’’. Ann Surg Oncol 7:296–300
Kanakoudis F, Petrou A, Michaloudis D, Chortaria G, Konstantinidou A (1996) Anaesthesia for intra-peritoneal perfusion of hyperthermic chemotherapy. Haemodynamic changes, oxygen consumption and delivery. Anaesthesia 51:1033–1036
Sakka SG, Ruhl CC, Pfeiffer UJ, Beale R, McLuckie A, Reinhart K, Meier-Hellmann A (2000) Assessment of cardiac preload and extravascular lung water by single transpulmonary thermodilution. Intensive Care Med 26:180–187
Vorgias G, Iavazzo C, Mavromatis J, Leontara J, Katsoulis M, Kalinoglou N, Akrivos T (2007) Determination of the necessary total protein substitution requirements in patients with advanced stage ovarian cancer and ascites, undergoing debulking surgery. Correlation with plasma proteins. Ann Surg Oncol 14:1919–1923
Desgranges FP, Steghens A, Mithieux O, Rosay H (2010) Potential risks of thoracic epidural analgesia in hyperthermic intraperitoneal chemotherapy. J Surg Oncol 101:442
Desgranges FP, Steghens A, Rosay H, Méeus P, Stoian A, Daunizeau AL, Pouderoux-Martin S, Piriou V (2012) Epidural analgesia for surgical treatment of peritoneal carcinomatosis: a risky technique? Ann Fr Anesth Reanim 31:53–59
Schmidt C, Creutzenberg M, Piso P, Hobbhahn J, Bucher M (2008) Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia 63:389–395
De Somer F, Ceelen W, Delanghe J, De Smet D, Vanackere M, Pattyn P, Mortier E (2008) Severe hyponatremia, hyperglycemia, and hyperlactatemia are associated with intraoperative hyperthermic intraperitoneal chemoperfusion with oxaliplatin. Perit Dial Int 28:61–66
Raft J, Parisot M, Marchal F, Tala S, Desandes E, Lalot JM, Guillemin F, Longrois D, Meistelman C (2010) Impact of the hyperthermic intraperitoneal chemotherapy on the fluid-electrolytes changes and on the acid-base balance. Ann Fr Anesth Reanim 29:676–681
Argenta PA, Sueblinvong T, Geller MA, Jonson AL, Downs LS Jr, Carson LF, Ivy JJ, Judson PL (2013) Hyperthermic intraperitoneal chemotherapy with carboplatin for optimally-cytoreduced, recurrent, platinum-sensitive ovarian carcinoma: a pilot study. Gynecol Oncol 129:81–85
Frenel JS, Leux C, Pouplin L, Ferron G, Berton Rigaud D, Bourbouloux E, Dravet F, Jaffre I, Classe JM (2011) Oxaliplatin-based hyperthermic intraperitoneal chemotherapy in primary or recurrent epithelial ovarian cancer: a pilot study of 31 patients. J Surg Oncol 103:10–16
Ansaloni L, Agnoletti V, Amadori A, Catena F, Cavaliere D, Coccolini F, De Iaco P, Di Battista M, Framarini M, Gazzotti F, Ghermandi C, Kopf B, Saponara M, Tauceri F, Vallicelli C, Verdecchia GM, Pinna AD (2012) Evaluation of extensive cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer 22(5):778–785
Tentes AA, Kakolyris S, Kyziridis D, Karamveri C (2012) Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy in the treatment of advanced epithelial ovarian cancer. J Oncol 2012:358341
Konigsrainer I, Beckert S, Becker S, Zieker D, Fehm T, Grischke EM, Lauk O, Glatzle J, Brücher B, Wallwiener D, Königsrainer A (2011) Cytoreductive surgery and HIPEC in peritoneal recurrent ovarian cancer: experience and lessons learned. Langenbecks Ach Surg 396:1077–1081
Fagotti A, Costantini B, Vizzielli G, Perelli F, Ercoli A, Gallotta V, Scambia G, Fanfani F (2011) HIPEC in recurrent ovarian cancer patients: morbidity-related treatment and long-term analysis of clinical outcome. Gynecol Oncol 122(2):221–225
Cascales Campos PA, Gil Martinez J, Galindo Fernandez PJ et al (2011) Perioperative fast track program in intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) after cytoreductive surgery in advanced ovarian cancer. Eur J Surg Oncol 37:543–548
Deraco M, Kusamura S, Virzì S, Puccio F, Macrì A, Famulari C, Solazzo M, Bonomi S, Iusco DR, Baratti D (2011) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: multi-institutional phase-II trial. Gynecol Oncol 122:215–220
Roviello F, Pinto E, Corso G, Pedrazzani C, Caruso S, Filippeschi M, Petrioli R, Marsili S, Mazzei MA, Marrelli D (2010) Safety and potential benefit of hyperthermic intraperitoneal chemotherapy (HIPEC) in peritoneal carcinomatosis from primary or recurrent ovarian cancer. J Surg Oncol 102:663–670
Pomel C, Ferron G, Lorimier G et al (2010) Hyperthermic intra-peritoneal chemotherapy using oxaliplatin as consolidation therapy for advanced epithelial ovarian carcinoma. Results of a phase II prospective multicentre trial. CHIPOVAC study. Eur J Surg Oncol 36:589–593
Ceelen WP, Van Nieuwenhove Y, Van Belle S, Denys H, Pattyn P (2009) Cytoreduction and hyperthermic intraperitoneal chemoperfusion in women with heavily pretreated recurrent ovarian cancer. Ann Surg Oncol 19:2352–2359
Lim MC, Kang S, Choi J, Song YJ, Park S, Seo SS, Park SY (2009) Hyperthermic intraperitoneal chemotherapy after extensive cytoreductive surgery in patients with primary advanced epithelial ovarian cancer: interim analysis of a phase II study. Ann Surg Oncol 16:993–1000
Bereder J, Glehen O, Habre J (2009) Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from ovarian cancer: a multiinstitutional study of 246 patients. J Clin Oncol 27(Suppl 15):Abstract 5542
Pavlov MJ, Kovacevic PA, Ceranic MS, Stamenkovic AB, Ivanovic AM, Kecmanovic DM (2009) Cytoreductive surgery and modified heated intraoperative intraperitoneal chemotherapy (HIPEC) for advanced and recurrent ovarian cancer—12-year single center experience. Eur J Surg Oncol 35:1186–1191
Guardiola E, Delroeux D, Heyd B, Combe M, Lorgis V, Demarchi M, Stein U, Royer B, Chauffert B, Pivot X (2009) Intra-operative intra-peritoneal chemotherapy with cisplatin in patients with peritoneal carcinomatosis of ovarian cancer. World J Surg Oncol 7:14
Di Giorgio A, Naticchioni E, Biacchi D, Sibio S, Accarpio F, Rocco M, Tarquini S, Di Seri M, Ciardi A, Montruccoli D, Sammartino P (2008) Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 113:315–325
Bae JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, Ahn WS, Namkoong SE (2007) Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol 106:193–200
Cotte E, Glehen O, Mohamed F, Lamy F, Falandry C, Golfier F, Gilly FN (2007) Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg 31:1813–1820
Helm CW, Randall-Whitis L, Martin RS 3rd, Metzinger DS, Gordinier ME, Parker LP, Edwards RP (2007) Hyperthermic intraperitoneal chemotherapy in conjunction with surgery for the treatment of recurrent ovarian carcinoma. Gynecol Oncol 105:90–96
Rufian S, Muñoz-Casares FC, Briceno J, Diaz CJ, Rubio MJ, Ortega R, Ciria R, Morillo M, Aranda E, Muntané J, Pera C (2006) Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol 94:316–324
Raspagliesi F, Kusamura S, Campos Torres JC, de Souza GA, Ditto A, Zanaboni F, Younan R, Baratti D, Mariani L, latterza B, Deraco M (2006) Cytoreduction combined with intraperitoneal hyperthermic perfusion chemotherapy in advanced/recurrent ovarian cancer patients: the experience of National Cancer Institute of Milan. Eur J Surg Oncol 32:671–675
Look M, Chang D, Sugarbaker PH (2004) Long-term results of cytoreductive surgery for advanced and recurrent epithelial ovarian cancers and papillary serous carcinoma of the peritoneum. Int J Gynecol Cancer 14:35–41
Ryu KS, Kim JH, Ko HS, Kim JW, Ahn WS, Park YG, Kim SJ, Lee JM (2004) Effects of intraperitoneal hyperthermic chemotherapy in ovarian cancer. Gynecol Oncol 94:325–332
Piso M, Dahlke MH, Loss M, Schlitt HJ (2004) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from ovarian cancer. World J Surg Oncol 2:21
Zanon C, Clara R, Chiappino I, Bortolini M, Cornaglia S, Simone P, Bruno F, De Riu L, Airoldi M, Pedani F (2004) Cytoreductive surgery and intraperitoneal chemohyperthermia for recurrent peritoneal carcinomatosis from ovarian cancer. World J Surg 28:1040–1045
Chatzigeorgiou K, Economou S, Chrysafis G, Dimasis A, Zafiriou G, Setzis K, Lyratzopoulos N, Minopoulos G, Manolas K, Chatzigeorgiou N (2003) Treatment of recurrent epithelial ovarian cancer with secondary cytoreduction and continuous intraoperative intraperitoneal hyperthermic chemoperfusion (CIIPHCP). Zentralbl Gynakol 125:424–429
de Bree E, Romanos J, Michalakis J, Relakis K, Georgoulias V, Melissas J, Tsiftsis DD (2003) Intraoperative hyperthermic intraperitoneal chemotherapy with docetaxel as second-line treatment for peritoneal carcinomatosis of gynaecological origin. Anticancer Res 23:3019–3027
Conflict of interest
Dr Villarejo has a Government grant for a Clinical Trial to compare plain surgery with surgery plus intraoperative hyperthermic paclitaxel chemotherapy. The device we used for this pilot series is the Combat PRS™ (Peritoneum Recirculation System, Galmaz Biotech, Madrid, Spain), borrowed at no cost by the owner.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pascual-Ramírez, J., Sánchez García, S., González Ruiz de la Herrán, F. et al. Security and efficiency of a closed-system, turbulent-flow circuit for hyperthermic intraperitoneal chemotherapy after cytoreductive ovarian surgery: perioperative outputs. Arch Gynecol Obstet 290, 121–129 (2014). https://doi.org/10.1007/s00404-014-3153-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3153-4