Abstract
Introduction
The objective of this study was to determine whether ovarian reserve markers can predict ovarian response in women undergoing their first cycle of assisted reproduction.
Materials and methods
This prospective observational study included 292 infertile patients undergoing their first IVF trial in the Assisted Reproductive Unit in a tertiary care hospital. Day 2 follicle stimulating hormone (FSH), Inhibin B, anti-Mullerian hormone (AMH), antral follicle count (AFC) and ovarian volume was measured before commencement of controlled ovarian hyperstimulation. The main outcome measures were oocytes retrieved and this was correlated with ovarian reserve markers.
Results
The mean age was 31.8 (±4.4) years and mean duration of infertility 8.2 (±3.9) years. The correlation between oocytes retrieved and age, day 2 FSH, Inhibin B, AMH, AFC and volume of the ovary was calculated. A negative correlation was found with age (r = −0.22, p < 0.001) and day 2 FSH (r = −0.35, p < 0.001). A positive correlation was seen with AMH (r = 0.15, p = 0.022), AFC (r = 0.48, p < 0.05) and volume (r = 0.17, p = 0.009). In the bivariate analysis, 1 year increase in age was found to decrease the oocytes retrieved count by 0.37 with a significant p value. The independent significant factors found in multiple linear regression analysis were day 2 FSH and AFC.
Discussion
The present study concludes that day 2 FSH and AFC are promising biomarkers for ovarian reserve in predicting ovarian response to gonadotropin stimulation in IVF patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian reserve in a woman describes her natural oocyte endowment and thereby reflects her reproductive potential. With changing social dynamics, women in recent times have elected to postpone childbearing until after the age of 35 years when there is an age-related downward trend in the total number of oocytes available for conception [1]. As a result, clinicians involved with the treatment of infertility face the arduous challenge of determining ovarian reserve to predict IVF outcome. The cost of treatment, discomfort a patient goes through due to multiple injections and the complications associated with the treatment per se does justify the need to clinically assess ovarian reserve before commencing treatment as poor response to ovarian stimulation is no longer acceptable. Passive assessment for ovarian reserve include age of the patient, basal hormone levels including follicle stimulating hormone (FSH), estradiol (E2) levels, serum inhibin B levels, anti-Mullerian hormone (AMH). Other surrogate markers include ultrasound determination of antral follicle count (AFC), ovarian volume and vascular resistance [2]. Dynamic tests such as gonadotropin-releasing hormone (GnRH) agonist test (GAST) and clomiphene challenge tests have also been used to predict the ovarian response to gonadotropin stimulation [3]. Amongst the available biomarkers interest has recently been generated in AMH as a reliable, accurate and reproducible predictor of ovarian reserve and IVF cycle outcome. Despite a wide range of ovarian reserve tests being available to clinicians, no single test provides a sufficiently accurate result. Whether these ovarian reserve markers have any added value to chronological age and pregnancy prospects still remains unclear. However, in current clinical practice most of these tests for ovarian reserve have low predictive accuracy and are better used for screening purposes. By screening these women for ovarian reserve one can decide using higher doses of gonadotropins in poor responders for better oocyte yield and without the fear of ovarian hyperstimulation syndrome (OHSS) [4].
A successful IVF procedure is one that translates into pregnancy and this is dependent on an adequate response of the ovaries when stimulated with exogenous gonadotropins [5]. Progressive depletion of the pool of ovarian reserve and age-related changes in oocytes are together responsible for this decline in reproductive potential in women [6]. Women with advanced chronological age have a higher probability of poor ovarian response but then even younger women may not be spared [7, 8]. A clinician may cancel an IVF cycle in a woman with poor ovarian response, a problem observed in 12–30 % of all women undergoing ovarian stimulation [9, 10]. The response to ovarian stimulation is highly variable in women of the same age group reflecting an inter-subject variation. Therefore, clinically it becomes imperative to identify that subset of women who are still young in age but have reduced ovarian reserve as well as those women whose fertility is hampered due to their advanced age. Once this can be achieved then management can be individualised and optimal stimulation regimens can be used in them while maximising their chances of conception.
The aim of this study was to determine whether ovarian reserve markers can predict ovarian response in women undergoing their first cycle of assisted reproduction. The main outcome measures were oocytes retrieved and this was correlated with ovarian reserve markers.
Materials and methods
This prospective study was conducted on 292 infertile patients undergoing their first IVF trial in the Assisted Reproductive Unit in a tertiary care hospital. This study was approved by the Institute’s Ethics Committee and all participating women were asked for their written consent. The study was conducted from September 2009 till September 2011. Women with no previous surgeries on the ovary/ovaries were included in the study. We excluded women with, PCOS, endometriosis, prior surgery and single ovary. The main outcome measures recorded were age and AFC, volume of ovaries as well as basal hormonal levels of FSH, Inhibin B, AMH and the number of oocytes retrieved.
The blood sample for the determination of FSH, Inhibin B and AMH was taken on the 2nd to 5th day of menstrual cycle and the IVF procedure was performed in the following month. The blood was obtained by vene puncture under strict aseptic condition. The blood was centrifuged at 3,500 rpm for 15 min and serum was stored in 1.5 ml eppendorf tubes at 20 °C. Serum FSH levels were determined by immune enzymometric assay ELISA technique using the EIAGEN FSH kit following the manufactures protocol. The analytical sensitivity was 0.6mIU/ml and intra assay and inter assay CV were <8.5 and <9.4 % respectively. Serum Inhibin B levels were determined by sandwich ELISA technique using the INHIBIN B DSL-10-84100i kit following the manufactures protocol. The analytical sensitivity was 7 pg/ml and intra assay and inter assay CV were <3.5 and <6.2 % respectively. Serum AMH was measured by EIA AMH/MIH kit (A Beckman Coulter Company) following the manufacturers protocol. For AMH the analytical sensitivity was 0.14 ng/ml and intra assay and inter assay CV were <12.3 and <14.2 % respectively.
A baseline pretreatment 3D ultrasound was done in the early follicular phase (days 2–5) of the woman’s spontaneous menstrual cycle for AFC and ovarian volume. We measured all follicles measuring 2–10 mm. The ultrasonographer was blinded to the results of the hormone assay. The intra-analysis coefficient of variation for follicular diameter measurements was <5 % and the lower limit of detection 0.5 mm. All the patients were started on a long down regulation protocol with gonadotropin releasing hormone (GnRH) analogue (1 mg leuprolide acetate subcutaneously) in the luteal phase of the same cycle. When enough suppression was achieved (E2 <50 pg/ml, LH <2 IU/ml and no ovarian cyst on ultrasound), the GnRH analogue dose was reduced by 50 % and controlled ovarian stimulation initiated with recombinant FSH. For each patient, the starting dose of recombinant FSH was individualised according to her age, BMI, AFC and FSH. The cycle was monitored based on the serum concentrations of estradiol on days 7 and 9 as well as transvaginal sonographic scan of the ovary and dose of FSH adjusted according to the response. The FSH dose remained same if E2 concentration varied between 1,000 and 4,500 pg/ml and was increased in case serum E2 level was <1,000 pg/ml. The dose of FSH was decreased if serum E2 levels exceeded 4,500 pg/ml. The total gonadotropin dose used was calculated. The stimulation was cancelled if no follicle developed or if E2 levels were <200 pg/ml even after 10 days of stimulation. Patients were administered 10,000 IU of human chorionic gonadotropin (HCG) when at least three follicles attained a diameter of 18 mm. Ovum pick up was carried out under intravenous sedation, 36 h after HCG injection, by transvaginal ultrasound-guided follicular aspiration. Embryo transfer was performed on day 3 using fresh embryos or a blastocyst transfer on day 5. All patients received luteal phase support with intramuscular administration of micronized progesterone for 2 weeks.
Statistical analysis
Data were presented as mean ± SD. The correlation between oocytes retrieved and ovarian reserve markers was calculated using Spearman Rank correlation coefficient. The factors associated with oocytes retrieved were identified using linear regression analysis. All the factors in the bivariate analysis were taken to the multiple linear regression analysis to find the independent factors associated with oocytes retrieved. The p value <0.05 was considered to be statistically significant.
Results
In this prospective observational study, we recruited 292 infertile women whose average age was 31.8 years (SD 4.4) with an average duration of infertility of 8.2 years (SD 3.9). Their BMI was 24.9 (SD 3.7) kg/m2. The ovarian reserve markers assessed of these women has been presented in Table 1. The oocytes retrieved after controlled ovarian hyperstimulation was found to be 9.9 (SD 7.2). In our study, 292 patients underwent 308 IVF/ICSI cycles. Eleven patients were cancelled due to poor response to stimulation (6) and failed fertilisation (5) with a cancellation rate of 3.8 %.
The correlation between oocytes retrieved and age, day 2–5 FSH, Inhibin B, AMH, AFC and volume of the ovaries was calculated. A negative correlation was found with age (r = −0.22, p < 0.001) (Fig. 1) and day 2–5 FSH (r = −0.35, p < 0.001) (Fig. 2) as shown in Table 2. A positive correlation was seen with AMH (r = 0.15, p = 0.022) (Fig. 3), AFC (r = 0.48, p < 0.05) (Fig. 4) and volume (r = 0.17, p = 0.009). We correlated the age and ovarian reserve markers associated with oocytes retrieved using linear regression analysis. In the bivariate analysis, 1 year increase in age was found to decrease the oocytes retrieved count by 0.37, which was significant. In this bivariate analysis other significant factors affecting oocyte retrieved were day 2–5 FSH, AMH, AFC and volume of the ovaries. The variation contributed by AFC (R 2 = 23.5 %) was the highest followed by FSH (R 2 = 10.2 %). The independent significant factors found in multiple linear regression analysis were day 2–5 FSH and AFC as shown in Table 3. Inhibin B was a mere factor that contributed to oocytes retrieved in these infertile women. The variation explained (R 2) by ovarian reserve markers on oocytes retrieved was 27 %.
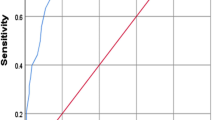
We found the cutoff value for these independent factors namely FSH and AFC for predicting poor oocytes retrieved (≤4) [9]. The cut off for day 2–5 FSH for predicting poor oocytes retrieved was 6.36 mIU/ml in our study with a sensitivity of 62.5 %, specificity of 59.6 % and area under the curve (AUC) of 66.5 % (Fig. 5). These infertile women with day 2–5 FSH >6.36 had a 2.5 times higher odds of having a poor oocyte recovery. Similarly for AFC we found a cut off of ≤13 for predicting poor oocytes retrieved with a sensitivity of 69.4 %, specificity of 58.4 % and AUC of 71.4 % (Fig. 6). Thus, women with AFC <13 had a 3.2 times higher chance of poor oocyte recovery.
Discussion
The data in this study indicates that a pretreatment day 2–5 FSH and AFC measured using 3D ultrasound are the most significant predictors of oocytes retrieved and poor ovarian response after gonadotropin stimulation in women undergoing their first cycle of assisted reproduction. Not all women respond well to controlled ovarian hyperstimulation. Before commencement of the IVF cycle if one assesses the ovarian reserve markers then poor responders can be identified and this may reduce the stress caused by cycle cancellation or low oocytes retrieved [11]. Subsequently, it may help the infertile couple to choose alternative methods of treatments like oocyte donation or even adoption and avoid the futility of repeated stimulation cycles. On the other hand, a pretreatment analysis of ovarian reserve may identify women who are at a higher risk for developing OHSS and such patients may be treated with lower doses of gonadotropins in a GnRH-antagonist cycle under careful monitoring keeping a low threshold for cycle cancellation or cryopreservation of all embryos with transfer in subsequent cycle [12, 13].
Previous studies have shown that a sharp decline in primordial follicles occurs at around 38 years of age [14]. The accelerated depletion of ovarian reserve is reflected by a rise in serum concentration of FSH and a reduction in serum concentration of AMH. By screening women for “early ovarian ageing” in their late third or early fourth decade of life may provide useful information about their reproductive potential and allow them to take rationale decision regarding their future fertility and plan for early conception or even cryopreservation of oocytes/embryos for later use. However, various test combinations have been tried but none of them has been successful in predicting which woman will experience early ovarian ageing or a woman’s performance in an IVF cycle [15].
It has been known from ancient times that women with advanced age have difficulty in achieving conception and this is still valid in modern era. Although age is an important parameter to assess fertility, we know that ovarian age may differ from chronological age and around 10 % of women may experience diminished ovarian reserve in their early to mid-30s [2]. In our prospective observational study, we correlated the age and ovarian reserve markers associated with oocytes retrieved using linear regression analysis. Univariate linear regression of square root of number of oocytes, based on model significance and R 2 were Age (R 2 = 0.049), day 2–5 FSH (R 2 = 0.102), AMH (R 2 = 0.025), AFC (R 2 = 0.235) and volume (R 2 = 0.0429). The independent significant factors found in multiple linear regression analysis were day 2–5 FSH and AFC while Inhibin B was a mere factor that contributed to oocytes retrieved in these infertile women. Further, in the bivariate analysis, we observed that 1 year increase in age was found to decrease the oocytes retrieved count by 0.37, which was statistically significant. In a retrospective study, patient’s age (R 2 = 0.215) and AFC (R 2 = 0.24) appeared good markers for predicting the total number of oocytes retrieved in women undergoing IVF treatment [16]. In our study, we found a positive correlation with AFC (r = 0.48, p < 0.05) and oocytes retrieved. Our findings were in agreement with the systematic review, where the authors examined the tests for ovarian reserve in predicting IVF outcome and suggested that AFC was the single most accurate predictor of poor ovarian reserve in women undergoing IVF [17].
In the present study AMH demonstrated an equivalent predictive ability as that of age, day 2–5 FSH, AFC and ovarian volume in the univariate analysis but this was not statistically significant in the multivariate analysis. AMH also known as Mullerian-inhibiting substance is a dimeric glycoprotein comprising of two monomers attached to each other by disulphide bonds. It belongs to the transforming growth factor-B super family, which acts on tissue growth and differentiation [18]. It is involved in the regression of the Mullerian ducts during embryonic development of a male fetus [19]. Females express AMH from the granulosa cells of the preantral and small antral follicles from birth up to menopause. Once the follicles differentiate from the primordial to the primary stage, AMH production begins and continues till the follicles have reached the antral stages with a diameter of 2–6 mm [20]. As age advances there is a decrease in the antral follicles and AMH production decreases becoming undetectable at and after menopause [21]. AMH represents the quantitative ovarian reserve and may have some predictive value for predicting pregnancy as well [22]. When compared to FSH, E2 and inhibin B, AMH is cycle-independent [23]. AMH is a promising biomarker for ovarian reserve as it indicates the quantity of oocytes but is not related to their quality. However, as a single parameter it has limited value in predicting ovarian response to gonadotropin stimulation in IVF patients. Our work differs from the previous study by Jayaprakasan et al. [24] where the authors concluded that AFC and AMH are the best predictors of poor response to ovarian stimulation during IVF. However, reliable cutoffs for AMH need to be defined. Marca et al. [25] evaluated the role of AMH as a predictive marker in assisted reproductive technology (ART) and found it to be a better marker in predicting the response of a woman to COH when compared to age, FSH, Estradiol and Inhibin B.
In the present study, there was a positive correlation seen with AMH (r = 0.15, p = 0.022) and oocytes retrieved using linear regression analysis. However, in the multiple linear regression analysis FSH and AFC were the independent significant factors that contributed to the number of oocytes retrieved. In a study by Anckaert et al. [26], the authors looked at the predictive value of serum follicular fluid levels of AMH with respect to ovarian response. Their study confirmed a positive relationship between serum AMH concentrations and ovarian response to COH in terms of oocytes retrieved and the endocrine response. In another study, Yates et al. [27] demonstrated that AMH-based COH regimens showed a statistically significantly improvement in positive clinical outcomes, with reduced incidence of complications and had a lesser financial burden in ART cycles. In the present study, our patients had a relatively low age with a relatively low SD (31.8 ± 4.4), which could perhaps explain the fact that age and AMH failed to be independent significant factors in the multiple regression analysis.
Antral follicle count directly measure the “selectable” follicle population while ovarian volume indirectly assesses the size of the follicles. In the present study, ovarian volume was predictive of the number of oocytes retrieved on univariate analysis, but with a predictive ability less than that of FSH and AFC. This is in agreement with a recent meta-analysis by Hendriks et al. [28].
When we evaluated the cutoff value for the independent factors namely FSH and AFC for predicting poor oocytes retrieved (≤4) we found that the cut off for FSH for predicting poor oocytes retrieved was 6.36 mIU/ml in our study with a sensitivity of 62.5 %, specificity of 59.6 % and AUC of 66.5 %. Thus, these infertile women with day 2–5 FSH of >6.36 had a 2.5 times higher odds of having a poor oocyte recovery. Likewise for AFC, we found a cut off of ≤13 for predicting poor oocytes retrieved with a sensitivity of 69.4 %, specificity of 58.4 % and AUC of 71.4 %, which implied that women with AFC <13 had a 3.2 times higher chance of poor oocyte recovery. In a study similar to ours, the authors concluded that AFC and AMH had the highest ability to differentiate between the poor responders from the normoresponders with an AUC of 93.5 and 90.5 %, respectively [24].
Our study has several limitations as it does not take pregnancy rate or live birth rate into account. The sample size in this study is small. Also, ovarian reserve markers have limited role in the prediction of nonconception and their use in routine clinical practice is questionable.
Conclusion
As a clinician one needs to identify that subset of patients who are likely to respond poorly during an IVF cycle. Ovarian reserve tests may help in the identification of such participants and it is these couples who need counselling as they have a higher chance of the cycle being cancelled and a low probability of achieving success in terms of pregnancy, thereby allowing them to make an informed decision. Therefore, the clinicians can individualise treatment protocols in these women to obtain maximum ovarian response.
References
Astolfi P, Ulizzi L, Zonta LA (1999) Selective cost of delayed childbearing. Hum Reprod 14:572–573
Johnson NP, Bagrie EM, Coomarasamy A, Bhattacharya S, Shelling AN, Jessop S, Farquhar C, Khan KS (2006) Ovarian reserve tests for predicting fertility outcomes for assisted reproductive technology: the international systematic collaboration of ovarian reserve evaluation protocol for a systematic review of ovarian reserve test accuracy. BJOG 113:1472–1480
Coccia ME, Rizzello F (2008) Ovarian reserve. Ann N Y Acad Sci 1127:27–30 (Review)
Arslan M, Bocca S, Mirkin S, Barroso G, Stadtmauer L, Oehninger S (2005) Controlled ovarian hyperstimulation protocols for in vitro fertilization: two decades of experience after the birth of Elizabeth Carr. Fertil Steril 84:555–569
Akande VA, Fleming CF, Hunt LP, Keay SD, Jenkins JM (2002) Biological versus chronological ageing of oocytes, distinguishable by raised FSH levels in relation to the success of IVF treatment. Hum Reprod 17:2003–2008
Sauer MV (1998) The impact of age on reproductive potential: lessons learned from oocyte donation. Maturitas 30:221–225
Nikolaou D, Templeton A (2003) Early ovarian ageing: a hypothesis. Detection and clinical relevance. Hum Reprod 18:1137–1139
Lambalk CB, van Disseldorp J, de Koning CH, Broekmans FJ (2009) Testing ovarian reserve to predict age at menopause. Maturitas 63:280–291
Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L (2011) ESHRE working group on poor ovarian response definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 26(7):1616–1624
Keay SD, Liversedge NH, Mathur RS, Jenkins JM (1997) Assisted conception following poor ovarian response to gonadotrophin stimulation. Br J Obstet Gynaecol 104:521–527
Mcllveen M, Skull JD, Ledger WL (2007) Evaluation of the utility of multiple endocrine and ultrasound measures of ovarian reserve in the prediction of cycle cancellation in a high-risk IVF population. Hum Reprod 22:778–785
Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, Mitchell P, Ambrose P, Fleming R (2009) Anti-Mullerian hormone-based approach to controlled ovarian stimulation for assisted conception. Hum Reprod 24:867–875
Mathur R, Kailasam C, Jenkins J (2007) Review of the evidence base of strategies to prevent ovarian hyperstimulation syndrome. Hum Fertil 10:75–85
Faddy MJ, Gosden RG (1995) A mathematical model of follicle dynamics in the human ovary. Hum Reprod 10:770–775
Muttukrishna S, McGarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P (2005) Antral follicle count, anti-Mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG 112:1384–1390
Lorusso F, Vicino M, Lamanna G, Trerotoli P, Serio G, Depalo R (2007) Performance of different ovarian reserve markers for predicting the number of oocytes retrieved and mature oocytes. Maturitas 56(4):429–435
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB (2006) A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 12:685–718
Fanchin R, Schonauer LM, Righini C, Frydman N, Frydman R, Taieb J (2003) Serum anti-Mullerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod 18:328–332
Behringer RR, Finegold MJ, Cate RL (1994) Mullerian-inhibiting substance function during mammalian sexual development. Cell 79:415–425
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA et al (2004) Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10:77–83
Van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH et al (2005) Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 83:979–987
Van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH et al (2002) Serum anti Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod 17:3065–3071
La Marca A, Stabile G, Artenisio AC, Volpe A (2006) Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod 21:3103–3107
Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N (2010) A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril 93(3):855–864
La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A (2010) Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 16(2):113–130
Anckaert E, Smitz J, Schiettecatte J, Klein BM, Arce J-C (2012) The value of anti-Mullerian hormone measurement in the long GnRH agonist protocol: association with ovarian response and gonadotrophin-dose adjustments. Hum Reprod 27:1829–1839
Yates AP, Rustamov O, Roberts SA, Lim HY, Pemberton PW, Smith A, Nardo LG (2011) Anti-Mullerian hormone-tailored stimulation protocols improve outcomes whilst reducing adverse effects and costs of IVF. Hum Reprod 26(9):2353–2362
Hendriks DJ, Kwee J, Mol BW, te Velde ER, Broekmans FJ (2007) Ultrasonography as a tool for the prediction of outcome in IVF patients: a comparative meta-analysis of ovarian volume and antral follicle count. Fertil Steril 87:764–775
Acknowledgments
The authors have not received any financial support for this study.
Conflict of interest
The authors do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N., Bahadur, A., Malhotra, N. et al. Prospective analysis of ovarian reserve markers as determinant in response to controlled ovarian stimulation in women undergoing IVF cycles in low resource setting in India. Arch Gynecol Obstet 288, 697–703 (2013). https://doi.org/10.1007/s00404-013-2802-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-2802-3