Abstract
Objective
To evaluate the role of serum level of VEGF-A in comparison to CA-125 in diagnosis and follow-up of patients with advanced endometriosis after conservative laparoscopic surgery.
Methods
A prospective randomized case–control study was performed on patients referred for laparoscopy complaining of unexplained primary infertility with or without chronic pelvic pain. Thirty patients with advanced endometriosis; stage III–IV were included (study group), another 30 women without endometriosis or any other medical conditions were settled as a control group. Pre-operative blood samples were collected from study and control cases. Post-operative blood samples were collected from 25 treated patients in the follicular phase of the third menstrual cycle; 5 cases were drop-outs. Serum level of cancer antigen-125 (CA-125) and vascular endothelial growth factor (VEGF-A) were assayed by using enzyme linked immunosorbent assay (ELISA) kit.
Results
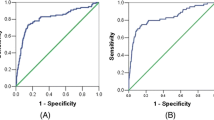
There was a statistically significant difference in serum CA-125 and VEGF-A level in patients with advanced endometriosis before conservative laparoscopic surgery and those without endometriosis (p < 0.001) and after conservative laparoscopic surgery (p < 0.001). High sensitivity (93.3 %), specificity (96.7 %) and accuracy (95.0 %) of VEGF-A assay than in CA-125 distinguishing between patients with endometriosis from those without endometriosis; CA-125 has 70.0 %sensitivity, 90.0 % specificity and 85.0 % accuracy. Percentage of decrease of VEGF-A level after operation was higher than that of CA-125 (45.9 vs. 25.8 %) p < 0.001, respectively.
Conclusion
The use of VEGF-A for diagnosis of advanced endometriosis at cut-off 680 pg/ml and for follow-up is better than CA-125.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis is a benign gynecologic disease defined as the presence of functional endometrial glands and stroma outside the uterine cavity, causing dysmenorrhea, dyspareunia, menstrual irregularities, and infertility [1]. Its general prevalence among all women of reproductive age is estimated to range between 3 and 10 % [2]. It can be classified into four stages: minimal, mild, moderate, and severe [3]. More advanced endometriosis can be deeply invasive or can present as ovarian endometriotic cysts [4]. The stage of endometriosis is positively correlated with the degree of sub-fertility, but not as clearly as with the degree of pelvic pain [5]. The gold standard for the diagnosis of endometriosis is laparoscopic inspection, ideally combined with histological confirmation [6].
The broad spectrum of clinical presentations of endometriosis has led to a wide variety of surgical and medical treatment options. The efficacy of medical and surgical treatment of endometriosis-associated infertility and pelvic pain is a source of ongoing controversy [7, 8].
One of the diagnostic markers already well established is cancer antigen-125 (CA-125), a high molecular weight glycoprotein (>20,000 Da) of epithelial origin that has been detected in normal and neoplastic epithelium of celomic origin such as endometrium, endocervix, epithelial cells of fallopian tubes and cancerous epithelial cells of the ovary [9, 10]. The first study of this marker in patients with endometriosis detected elevated serum levels [11].
Angiogenesis is a biological process through which new blood vessels develop from the endothelium of a preexisting vasculature and is likely involved in the pathogenesis of endometriosis [12]. Angiogenesis is induced by various peptide growth factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor, and thymidine phosphorylase [13, 14].
VEGF is the most prominent and most studied pro-angiogenic factor in endometriosis and it is widely believed that VEGF is the main stimulus for angiogenesis and increased vessel permeability in this disease [15]. VEGF-A belongs to a family of dimeric glycoproteins such as VEGF-B, -C, -D, -E and placental growth factor (PlGF). VEGF-A production is stimulated by cytokines, hormones, growth factors and hypoxia. One study observed that patients with endometriosis compared with healthy women had higher VEGF serum levels in the secretory phases and higher levels close to statistical significance in the proliferative phases, suggesting a role for angiogenesis in the pathogenesis of endometriosis [16], and it is proposed that the inhibition of VEGF may be an attractive novel therapeutic approach for the treatment of endometriosis [17].
VEGF-A exerts its biologic effects by binding to VEGF receptors 1 and 2 (VEGFR-1 and VEGFR-2). These two tyrosine kinase receptors also exist in soluble forms, sVEGFR-1 and -2, which can be quantified in body fluids such as peritoneal fluid and blood [18]. The soluble receptors are thought to act as negative regulators of VEGF availability for endothelial cells [19], thereby reducing angiogenic activity. Some studies have shown elevated levels of VEGF in the serum and peritoneal fluid of patients with endometriosis but they used specific control groups [20]. In this study we aimed to evaluate the role of serum level of VEGF-A in comparison to CA-125 in diagnosis and detection of recurrence of patients, with advanced endometriosis after conservative laparoscopic surgery.
Patients and methods
This prospective randomized case–control study was conducted from April 2008 to August 2010 at Cytogenetic and Endoscopy Unit, Obstetrics and Gynecology Department, Zagazig University Hospitals, Egypt. Informed written consent was taken from each participant before enrollment in the study and the study protocol was approved by the local ethics and research committee.
Patients
Patients were selected from those referred for laparoscopy complaining of unexplained primary infertility with/without chronic pelvic pain. Endometriosis was diagnosed during the laparoscopic procedure according to the morphological criteria [21] and confirmed by histopathological examination. The disease was staged according to the revised American Society for Reproductive Medicine (ASRM) classification [3]. Laproscopy was performed in the follicular phase of the cycle. Thirty patients with advanced endometriosis stage III–IV were included (study group); another 30 women without endometriosis or any other medical conditions were settled as a control group. All women enrolled in the study fulfilled the following inclusion criteria: (1) regular menses (defined by the presence of cycles lasting between 28 and 35 days in the previous year, with 2–7 days of flow) and (2) in the follicular phase of the cycle at the time of surgery. Exclusion criteria were: (1) hormonal treatment received 3 months prior to surgery, (2) history of ovarian cancer, (3) signs of ovarian failure, (4) patients with pelvic inflammatory disease or other gynecological pathologies, (5) previous pelvic surgery, (6) obese patients; BMI ≥ 30 kg/m2 and (7) smokers.
Methods
Collection of samples
Blood (serum) samples were collected in the morning before surgery from referred cases with unexplained primary infertility. Laparoscopy was done and all recognizable endometriotic lesions were treated by bipolar coagulation, resection of endometriotic nodules and ovarian cystectomy. Pre-operative blood samples were preserved for 30 patients with advanced endometriosis and 30 patients without endometriosis. Post-operatively, in the follicular phase of the third menstrual cycle, blood samples were collected from 25 treated patients; 5 cases were drop-outs. Five ml of venous blood was withdrawn into non-heparinised tubes. After keeping at room temperature for 30 min, blood samples were centrifuged for 15 min at 3000 rpm. Serum was obtained, aliquots were made and stored at −70 °C until assayed for VEGF and CA 125. VEGF was measured by Human VEGF Quantikine ELISA Kit (DVE00, R&D Systems, Minneapolis, MN) and CA 125 levels were measured by using ELISA kit for Can-Ag CA125 (Fujirebio Diagnostics, Inc., Goteborg, Sweden) and the expected value of this marker was 5.06–47.9 U/ml. These assays were analyzed according to manufacturer’s instructions.
The statistical analysis was performed using SPSS version 14 (SPSS, Chicago, IL, USA). Qualitative data were expressed as numbers and percentages and compared using the χ2 or fisher exact test when appropriate. Nonparametric quantitative data such as CA-125 and VEGF-A levels were expressed as mean ± SD and median and were compared using Mann–Whitney U test. Paired T-test was used for comparison of their levels before and after operation. Validation of tests were performed. p < 0.05 was considered statistically significant.
Results
Initially 30 patients with laproscopically proved advanced endometriosis were included as a study group, 5 patients lost follow up after conservative laproscopic surgery and were excluded from the study, another 30 women without endometriosis served as a control group. There was no statistically significant difference regarding age, symptoms, dysmenorrhea and history of acute pelvic infection between patients with advanced endometriosis and those without endometriosis. However, chronic pelvic pain was significantly higher in patients with advanced endometriosis than in those without endometriosis (p < 0.5) (Table 1).
Table 2 shows that there is a statistically significant difference in serum CA 125 level in patients with advanced endometriosis before laparoscopic conservative surgery and those without endometriosis [36.4 ± 14.2 vs. 12.6 ± 6.1] (p < 0.001) and after conservative laparoscopic surgery [27.0 ± 4.8 vs. 12.6 ± 6.1] (p < 0.001).
Also, there was a statistically significant difference in serum VEGF level in patients with advanced endometriosis before laparoscopic conservative surgery and those without endometriosis[735.1 ± 100.6 vs. 97.1 ± 12.1] (p < 0.001) and after conservative laparoscopic surgery [397.7 ± 55.0 vs. 97.1 ± 12.1] (p < 0.001) (Table 3 ). Percentage of decrease of VEGF level after operation showed a highly significant difference than that for CA-125 (45.9 vs 25.8 %) for the former and the later respectively (Table 3 ).
Table 4 shows the sensitivity and specificity and accuracy of CA-125 and VEGF-A for diagnosis of patients with advanced endometriosis (70, 90, 85.0 %) for the first and (93.3, 96.7, 95.0 %) for the second.
Discussion
The diagnosis of endometriosis is difficult and delayed because of the non-specific symptoms, late presentation and the use of laparoscopy, which is limited by available funding, the surgeon’s experience and its invasive nature. This may result in social and/or work-related problems for the patient, in addition to increased anxiety and fear of having a more serious condition [22].
Moreover, two-thirds of women who undergo laparoscopy for pelvic pain or infertility will be subjected to the potential risks as well as the cost associated with this procedure without actually having endometriosis [23].
The development of a simple blood test as a marker for screening of endometriosis would reduce the number of unnecessary interventions and would therefore be very useful [24]. The quest to develop a diagnostic test of endometriosis has mostly concentrated on the levels of CA-125 and cytokines [25]. However, none of them showed both high sensitivity and specificity in the diagnosis and follow-up of endometriosis. Hence, in this study we aimed to evaluate the role of serum level of VEGF-A in comparison to CA-125 in diagnosis and follow up of patients with advanced endometriosis after conservative laparoscopic surgery.
Our study showed that there was a statistically significant difference in serum CA-125 level in patients with advanced endometriosis before laparoscopic conservative surgery and those without endometriosis (p < 0.001).
Our observation of a correlation between raised CA-125 levels and endometriosis runs in agreement with previous studies [26–28]. Moreover, others have indicated that CA-125 may be more accurate at diagnosing women with later stages of disease [29–31]. The elevation of this marker in women with endometriosis is due to its higher concentration in ectopic than in entopic endometrium. Moreover, it may be due to inflammatory reactions, which alter endothelial permeability, thereby allowing the marker to reach the circulation [32].
In this study, we observed that preoperative serum VEGF-A level in patients with advanced endometriosis was significantly higher than those without endometriosis (p < 0.001).
Our finding runs in agreement with previous studies in which advanced endometriosis was associated with high level of VEGF-A in the serum [33] and peritoneal fluid [34] compared with healthy control.
Increase of VEGF may be due to immune system alterations that play an important role in the development of endometriosis. Locally, there is an immunoinflammatory reaction within the peritoneal cavity of endometriosis patients in which activated immune cells, together with endometriotic implants, produce high amounts of cytokines, growth factors, and angiogenic substances [35]. Moreover, as known, endometriosis is an estrogen-driven disease. Estradiol is found in high concentrations in endometriotic lesions [36]. Estrogen is a potent stimulus of angiogenesis through a direct increase of VEGF expression [37].
In addition, the extent of endometriosis and the mitotic activity in the lesion seems to be reflected by the levels of angiogenic growth factors in serum and peritoneal fluid compared with healthy women [17, 38]. Also, various polymorphisms in the VEGF gene have been associated with an increased risk of endometriosis [39, 40].
In contrast, other studies found no significant differences in serum levels of VEGF between women with and without endometriosis and concluded that diagnosis of endometriosis by laboratory testing of VEGF is not possible [41].
However, the fact that our patients had advanced endometriosis could explain the significant difference observed in the present study in contrast to previous study.
On comparing the validity of using either VEGF-A or CA-125 in diagnosis of patients with advanced endometriosis, we found a higher sensitivity, specificity and accuracy of VEGF-A assay than CA-125 in distinguishing between patients with endometriosis from those without endometriosis; [93.3, 96.7, and 95.0 %] versus [70.0, 90.0 and 85.0 %] for the former and the latter, respectively at a cut-off 680 pg/ml for VEGF-A and 35 μg/ml for CA-125.
The low specificity (90.0 %) of CA-125 in diagnosis of endometriosis by finding that 10 % of patients without endometriosis has high CA-125 serum level may be due to subclinical pelvic infection.
In addition, the low sensitivity (70.0 %) of CA-125 by finding that 30 % in patients with endometriosis has a normal CA-125 serum level could be explained by superficial location of endometriotic lesions.
We found that after conservative laparoscopic surgery, serum levels of both CA-125 and VEGF-A showed a statistically significant decrease, However, there was a highly significant decrease in VEGF-A level (45.9 %) compared to that of CA-125 (25.8 %).
This was in agreement with Bourlev et al. who found that surgical removal of endometriotic lesions resulted in decreased serum levels of pro-angiogenic VEGF-A [17].
So, we conclude that the use of VEGF-A for diagnosis of advanced endometriosis at a cut-off 680 pg/ml is better than CA-125. The addition of vascular endothelial growth factor (VEGF) to the diagnostic tool in cases with suspected widespread endometriosis might contribute to the accuracy of the diagnosis, sparing intensive endoscopic search for disseminated foci. It could also be used for the follow-up of those patients after conservative surgery.
The presence of VEGF inhibitors like soluble truncated receptor (sFlt-1) with affinity-purified antibody against VEGF is recommended in the treatment of endometriosis.
References
Giudice LC, Kao LC (2004) Endometriosis. Lancet 364:1789–1799
McLeod BS, Retzloff MG (2010) Epidemiology of endometriosis: an assessment of risk factors. Clin Obstet Gynecol 53:389–396
Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–21
Chopin N, Ballester M, Borghese B, Fauconnier A, Foulot H et al (2006) Relation between severity of dysmenorrhea and endometrioma. Acta Obstet Gynecol Scand 85:1375–1380
Fauconnier A, Chapron C (2005) Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update 11:595–606
Kennedy S, Bergqvist A, Chapron C, D’Hooghe T, Dunselman G et al (2005) ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod 20:2698–2704
Practice Committee of the American Society for Reproductive Medicine (2006) Treatment of pelvic pain associated with endometriosis. Fertil Steril 86:S18–S27
Practice Committee of the American Society for Reproductive Medicine (2006) Endometriosis and infertility. Fertil Steril 86:S156–S160
Barbieri RL, Niloff JM, Bast RC Jr, Scaetzl E, Kistner RW et al (1986) Elevated serum concentrations of CA-125 in patients with advanced endometriosis. Fertil Steril 45(5):630–634
Koninckx PR (1994) Is mild endometriosis a condition occurring intermittently in all women? Hum Reprod 9(12):2202–2205
Niloff JM, Knapp RC, Schaetzl E, Reynolds C, Bast RC Jr (1984) CA125 antigen levels in obstetric and gynecologic patients. Obstet Gynecol 64(5):703–707
Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M (1998) Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod 13:1686–1690
Gordon JD, Mesiano S, Zaloudek CJ, Jaffe RB (1994) Vascular endothelial growth factor localization in human ovary and fallopian tubes: possible role in reproductive function and ovarian cyst formation. J Clin Endocrinol Metab 81:353–359
Folkman J, Shing Y (1992) Angiogenesis. J Biol Chem 267:10931–10934
Taylor RN, Lebovic DI, Mueller MD (2002) Angiogenic factors in endometriosis. Ann NY Acad Sci 955:89–100
Xavier P, Belo L, Beires J, Rebelo I, Martinez-de-Oliveira J, Lunet N, Barros H (2006) Serum levels of VEGF and TNF-alpha and their association with C-reactive protein in patients with endometriosis. Arch Gynecol Obstet 273(4):227–231
Bourlev V, Volkov N, Pavlovitch S, Lets N, Larsson A et al (2006) The relationship between micro vessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction 132:503–511
Barleon B, Reusch P, Totzke F, Herzog C, Keck C et al (2001) Soluble VEGFR-1 secreted by endothelial cells and monocytes is present in human serum and plasma from healthy donors. Angiogenesis 4:143–154
Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Nordén-Lindeberg S et al (2007) Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol 109:1368–1374
Gagne D, Page M, Robitaille G, Hugo P, Gosselin D (2003) Levels of vascular endothelial growth factor (VEGF) in serum of patients with endometriosis. Hum Reprod 18:1674–1680
Ballard K, Lowton K, Wright J (2006) What’s the delay? A qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril 86:1296–1301
Donnez J, Squifflet J, Casanas-Roux F, Pirard C, Jadoul P et al (2003) Typical and subtle atypical presentations of endometriosis. Obstet Gynecol Clin North Am 30:83–93
Socolov R, Butureanu S, Angioni S, Sindilar A, Boiculese L et al (2011) The value of serological markers in the diagnosis and prognosis of endometriosis: a prospective case–control study. Eur J Obstet Gynecol Reprod Biol 154(2):215–217
Garry R (2006) Diagnosis of endometriosis and pelvic pain. Fertil Steril 86:1307–1309
Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M et al (2002) Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod 17:426–431
Amaral VF, Ferriani RA, Sa MF, Nogueira AA, Rosa e Silva JC et al (2006) Positive correlation between serum and peritoneal fluid CA-125 levels in women with pelvic endometriosis. Sao Paulo Med J 124:223–227
Martinez S, Garrido N, Coperias JL, Pardo F, Desco J et al (2007) Serum interleukin-6 levels are elevated in women with minimal-mild endometriosis. Hum Reprod 22:836–842
Rosa e Silva AC, Rosa e Silva JC, Ferriani RA (2007) Serum CA-125 in the diagnosis of endometriosis. Int J Gynaecol Obstet 96:206–207
Mol BW, Bayram N, Lijmer JG, Wiegerinck MA, Bongers MY et al (1998) The performance of CA-125 measurement in the detection of endometriosis: a meta-analysis. Fertil Steril 70:1101–1108
Somigliana E, Vigano P, Tirelli AS, Felicetta I, Torresani E et al (2004) Use of the concomitant serum dosage of CA 125, CA 19–9 and interleukin-6 to detect the presence of endometriosis. Results from a series of reproductive age women undergoing laparoscopic surgery for benign gynaecological conditions. Hum Reprod 19:1871–1876
Agic A, Djalali S, Wolfler MM, Halis G, Diedrich K et al (2008) Combination of CCR1 mRNA, MCP1, and CA125 measurements in peripheral blood as a diagnostic test for endometriosis. Reprod Sci 15:906–911
Seeber B, Sammel MD, Fan X, Gerton GL, Shaunik A et al (2008) Panel of markers can accurately predict endometriosis in a subset of patients. Fertil Steril 89:1073–1081
Bourlev V, Iljasova N, Adamyan L, Larsson A, Olovsson M (2010) Signs of reduced angiogenic activity after surgical removal of deeply infiltrating endometriosis. Fertil Steril 94:52–57
Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I et al (2004) Higher activity by opaque endometriotic lesions than nonopaque lesions. Acta Obstet Gynecol Scand 83:375–382
Harada T, Iwabe T, Terakawa N (2001) Role of cytokines in endometriosis. Fertil Steril 76:1–10
Bulun SE, Yang S, Fang Z, Gurates B, Tamura M et al (2002) Estrogen production and metabolism in endometriosis. Ann NY Acad Sci 955:75–85
Hyder SM, Nawaz Z, Chiappetta C, Stancel GM (2000) Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res 60:3183–3190
Bedaiwy MA, Falcone T (2004) Laboratory testing for endometriosis. Clin Chim Acta 340:41–56
Bhanoori M, Arvind Babu K, Pavankumar Reddy NG, Lakshmi Rao K, Zondervan K et al (2005) The vascular endothelial growth factor (VEGF) +405GNC 5′-untranslated region polymorphism and increased risk of endometriosis in South Indian women: a case control study. Hum Reprod 20:1844–1849
Kim SH, Choi YM, Choung SH, Jun JK, Kim JG et al (2005) Vascular endothelial growth factor gene +405 C/G polymorphism is associated with susceptibility to advanced stage endometriosis. Hum Reprod 20:2904–2908
Pupo-Nogueira A, de Oliveira RM, Petta CA, Podgaec S, Dias JA Jr et al (2007) Vascular endothelial growth factor concentrations in the serum and peritoneal fluid of women with endometriosis. Int J Gynaecol Obstet 99(1):33–37
Conflict of interest
We have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamed, M.L., El Behery, M.M. & Mansour, S.A.EA. Comparative study between VEGF-A and CA-125 in diagnosis and follow-up of advanced endometriosis after conservative laparoscopic surgery. Arch Gynecol Obstet 287, 77–82 (2013). https://doi.org/10.1007/s00404-012-2539-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-012-2539-4