Abstract
Objective
This study aimed to identify the effect of various risk factors as the promoters of HPV infection, and to identify which HPV-positive women may have an increased risk of developing cervical cancer.
Methods
Smear preparations were examined and classified according to the Bethesda system. HPV-DNA detection and genotyping was carried out using polymerase chain reaction combined with reverse hybridization line-probe assays. Age, smoking habit, age at first sexual intercourse, number of sexual partners, number of term births, contraceptive method, progesterone therapy, history of sexually transmitted diseases, history or existence of warts and existence of cervical infection were recorded.
Results
642 women (96 women with abnormal cervical cytology and 546 women with normal cytology) provided cervical samples. Smoking habit, number of sexual partners, number of term births, history of sexually transmitted diseases, history or existence of warts and existence of cervical infection were identified as the promoters of HPV infection. History of sexually transmitted diseases, history or existence of warts and existence of cervical infection were identified as cofactors affecting progression from HPV infection to cervical cancer. Neither of contraceptive methods studied was related to HPV infection or coexistence with malign transformation to cervical cancer.
Conclusion
Information gathered from this study could be used to prioritize limited screening and treatment services to woman who have specific characteristics that may put them at an increased risk of HPV infection. Additionally, by identifying which women have a higher risk of cervical cancer; it may be possible to reduce the number of unnecessary colposcopies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human papillomavirus (HPV) infection causes virtually all cases of cervical cancer (CC) and a less-defined, smaller fraction of vaginal, vulvar, penile, and anal cancers. Sexual contact with an infected partner is necessary for transmission of HPV infection, presumably through microscopic tears in the mucosa or skin [1]. The age of woman, the age at first sexual intercourse, barrier contraceptive use, co-infections, male sexual behavior, and male circumcision are other established risk factors for HPV infection [2].
Investigations have demonstrated that a range from 2% to more than 20% of the world’s female population have detectable HPV-DNA in their cervix at any time. Further, it has been established that, under certain conditions, these infections persist and are able to induce CC. The major steps known to be necessary in cervical carcinogenesis include HPV infection, HPV persistence over a certain period of time, progression to precancer, and invasion [3]. HPV infection alone may not be sufficient to cause CC, and other factors might exist that, in conjunction with HPV, influence the risk of progression to CC. High parity, smoking, and long-term use of oral contraceptives have been established as risk cofactors for CC among women with persistent infections with HPV. Other sexually transmitted diseases such as Chlamydia trachomatis, herpes simplex virus type 2, and human immunodeficiency virus as well as some poorly known dietary factors are likely intervening factors [2, 3].
Estimates of the prevalence of HPV infection vary greatly around the world, so the factors that contribute to the rare occurrence of CC after HPV infection might also differ from country to country. The aims of the present study are to identify the effect of various risk factors as the promoters of HPV infection and to identify which HPV-DNA positive women may have an increased risk of developing CC among a sample of women in our country.
Materials and methods
Study design
The study was conducted in accordance with the ethical principles stated in the most recent version of the Declaration of Helsinki. Prospectively planned data collection of 642 patients (aged 15–65 years) who attended cervical cancer screening service between January 2006 and January 2009 were evaluated in the study.
Women who were included in the study were interviewed to obtain information about their potential risk factors. The following data were collected: (1) demographic data, (2) reproductive and sexual histories, (3) risk factors for HPV and CC, and (4) past use of the CC screening program. Interviewed risk factors were smoking habit, the age at first sexual intercourse, number of sexual partners, number of term birth, contraceptive method (oral contraceptives, intrauterine contraceptive device), progesterone therapy, the history of sexually transmitted diseases, history or existence of warts, and existence of a cervical infection. Age at first sexual intercourse was examined using three groups: (1) less than 18, (2) between 18 to 25 and (3) aged 25 and older. The respondent’s number of lifetime sexual partners and number of term births were also modeled as a categorical variable, using two groups: one partner versus two or more.
A clinician obtained a vaginal and cervical specimen during the pelvic examination and performed a colposcopy evaluation. Cervical exfoliated cells were collected from all subjects by sampling the ecto- and endocervix using a cytobrush or spatula. All lesions observed during the colposcopic evaluations were biopsied, and in some cases an endocervical curetage was performed when the examination was not satisfactory. Smear preparations were examined and classified according to the Bethesda system [4]. The presence of HPV-DNA was assessed using a multiplex polymerase chain reaction (PCR) system. The amplified fragments of HPV-DNA positive samples were further analyzed by a highly sensitive, broad spectrum PCR and subsequently genotyped using reverse hybridization in a line probe assay [5, 6]. Nineteen types of high-risk HPV (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) and four types of low-risk HPV (HPV 6, 11, 42, 44) were classified.
HPV-DNA positive cases with normal cervical cytology were compared to HPV-DNA negative controls to examine the association between risk factors and risk of developing HPV infection. The purpose of this analysis was to identify the effect of these risk factors as the promoters of HPV infection.
High-risk HPV positive cases with abnormal cervical cytology were compared to high-risk HPV-DNA positive cases with normal cervical cytology to examine the association between the risk factors and risk of developing CC. The purpose of this analysis was to identify which HPV-DNA positive women may have an increased risk of developing CC.
Statistical analysis
Data were analyzed using the statistical package for Social Sciences 16.0 for Windows (SPSS Inc., Chicago, IL). The analysis of the data was obtained using descriptive statistical, Chi square test, Student’s t test, one way ANOVA and paired t test. Stepwise multiple regression analyses were also applied. The results were assessed within a 95% reliance and at a level of p < 0.05 significance.
Results
This study cohort included 642 females (mean age 3,606 ± 9,472 years; range 15–65 years). Multiplex PCR testing revealed 250 (38.9%) HPV-DNA positive cases and 392 (61.1%) HPV-DNA negative cases. The amplified fragments of 250 HPV-DNA positive samples genotyped using reverse hybridization showed high-risk HPV in 199 cases and low-risk HPV in 51 cases. Of these 199 high-risk HPV-positive cases, normal cervical cytology was observed in 130 cases and abnormal cervical cytology in 69 cases. Concerning 250 HPV-DNA positive cases, normal cervical cytology was observed in 175 cases and abnormal cervical cytology in 75 cases. A total of 546 (84.9%) women with normal cytology, 9 (1.55%) women with ASCUS, 4 (0.6%) women with ASC-H, 34 (5.3%) women with low-grade squamous intraepithelial lesions (LSIL), 26 (4.04%) women with high-grade squamous intraepithelial lesion (HSIL), and 23 (3.5%) women with squamous CC participated.
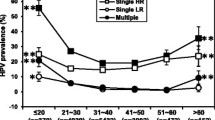
The distribution of risk factors associated with HPV infection was as follows: 321 patients (50%) smoked, but only 20% of those patients smoked for more than 10 years. Age at first coitus was under 18 years in 149 patients (23.1%), between 18 and 25 years in 427 patients (66.6%), and over 25 years in 66 patients (10.3%). 462 patients (72%) had a sexual partner, while 180 patients (28%) had more than one partner. 128 patients (19.9%) were nulliparous, and the remaining 514 patients (80.1%) were multiparous. While 260 patients (40.5%) did not use any contraception method, RIA (intrauterine device) was the most preferred method and used by 202 patients (31.5%). 578 patients (90%) did not report a history of sexually transmitted disease, whereas 64 patients (10%) had a history of sexually transmitted disease. 522 patients (81.2%) revealed no presence or history of condyloma, whereas the presence or history of condyloma was detected in 121 patients (18.8%). HPV-DNA positive cases with normal cervical cytology were compared to HPV-DNA negative controls to examine the association between risk factors and risk of developing HPV infection (Table 1). Smoking habit, number of sexual partners, number of term births, history of sexually transmitted diseases, history or existence of warts and existence of cervical infection were identified as the promoters of HPV infection. High-risk HPV-DNA positive cases with normal cervical cytology were compared to high-risk HPV-DNA positive cases with abnormal cervical cytology to examine the association between the risk factors and risk of developing CC (Table 2). History of sexually transmitted diseases, history or existence of warts and existence of cervical infection were identified as cofactors affecting progression from HPV infection to CC. Neither of the contraceptive methods studied was related to HPV infection or coexistence with malign transformation to CC. When concerning all the study group, hormonal contraceptives was not associated with abnormal cervical cytology (Table 3).
Discussion
In this study, the foci were on cofactors involved in the natural history of HPV infection and on cofactors affecting progression from HPV infection to CC. Our findings indicate that smoking habit, number of sexual partners, number of term births, history of sexually transmitted diseases, history or existence of warts and existence of cervical infection were associated with HPV infection while history of sexually transmitted diseases, history or existence of warts and existence of cervical infection were associated with progression from HPV infection to CC.
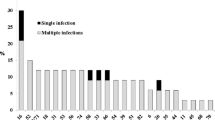
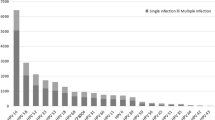
Until now, more than 100 HPV types have been identified and fully sequenced [7]. Approximately 40 HPV types infect the anogenital tract and a few are found in anogenital cancer biopsy specimens, notably CC. Oncogenic HPVs have been identified in almost all CC biopsies, i.e. 99.7% cases [8], what makes them undoubtedly the cause of the disease. Fifteen HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82 are considered as highly oncogenic (high-risk HPV), and HPV types 26, 53 and 66 as probably oncogenic, while HPV types 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81 and CP6108 are classified as viruses with low oncogenic potential (low-risk HPV) [9]. HPV 16 and 18 alone are found in nearly 60 and 17% of CC cases worldwide, respectively, while all other HPV types are found in less than 1–7% cases each [10]. In addition, multiple HPV infections seem to be very common and in previous studies were found in almost 7% of women with precancerous cervical lesions [11]. In our study, HPV-DNA detection and genotyping was carried out using PCR combined with reverse hybridization line-probe assays. High-risk HPV was classified in 199 cases and low-risk HPV in 51 cases.
Ideally, if we accept the premise that all CCs are caused by HPV infection, an assessment of risk factors requires a study group known to be exposed to HPV. From that HPV-exposed group, the added risk attributable to other factors can be estimated. Identifying the factors that contribute to the development of CC after HPV infection is very important because most women who receive a positive HPV test result do not go on to develop disease. The results from this study indicate that certain factors, such as history of sexually transmitted diseases, history or existence of warts and existence of cervical infection were associated with an increased risk of developing CC among high-risk HPV positive women.
Of four case–control studies, smoking was found to be a significant risk factor for squamous cell carcinoma but not for adenocarcinoma in two [12, 13], while no statistically significant association was evident between smoking and CC in the other two studies [14, 15]. In this study, smoking was associated with HPV infection but not with the risk of carcinoma of the cervix. The lack of association between smoking and risk of CC in this study may be due to the fact that even 50% of the respondents reported smoking, only 20% reported having smoked for more than 10 years.
Woman who had sexual intercourse for the first time at a younger age may have a greater number of sexual partners and may have been exposed to a persistent HPV infection for a longer time than women who began to have sex at a later age [16]. Early age at first intercourse, while clearly related to lifetime number of partners, is generally considered to be an independent risk factor for both HPV infection and carcinoma of the cervix [16]. However, studies that found no association between age at first intercourse and cancer risk in analyses adjusted for the number of sexual partners are also exist in the literature [13, 14, 17]. In this study, both age of woman at first sexual intercourse and number of sexual partners were associated neither with developing HPV infection nor with progression from HPV infection to CC. This could be explained, in part, because most of the women in our study reported to have sexual intercourse after the age of 18 and also a low number of lifetime sexual partners.
Munoz et al. [18] in a pooled analysis of 10 case–control studies in HPV-DNA positive women found that the risk of CC increased with the number of pregnancies. Some case–control studies have shown an association with parity for carcinoma of the cervix [17, 19–21]. Altekruse et al. [17] found an inverse relationship between parity and cervical carcinoma risk. No association with parity for either adenocarcinoma or squamous cell carcinoma of the cervix was found in two cohort studies [22, 23]. In this study, high parity was associated with HPV infection but not with the risk of carcinoma of the cervix.
Oral contraceptive use has been found to be associated with CC in many, but not in all studies. Recent analyses of case–control and cohort studies have confirmed that risk for CC is directly related to the duration of use of oral contraceptives in all and in HPV-DNA positive women [24, 25]. The effect of oral contraceptives on cancer risk is strongest for current and recent use and decreases with time since last use [26]. There are also studies with findings inconsistent with those from other studies showing no association between the use of oral contraceptives and risk of CC [13]. This study did not find an association between use of hormonal contraception and risk of developing HPV infection or CC. The lack of association may be explained by the fact that most studies report an increased risk of CC among long-term users of oral contraceptives. Less than 11% of this study participants reported using a hormonal contraceptive method. The use of barrier methods of contraception has been associated with decreased risk both of preinvasive cervical lesions and of CC [27], and this association has been found in studies restricted to HPV-DNA positive women [21, 28, 29]. The evidence, although suggestive of a protective effect of barrier contraception, is not entirely consistent and interpretation is severely limited in most studies by the lack of detailed reliable evidence on contraceptive use. Our results showed that barrier contraceptives are not associated with developing HPV infection or CC. Use of progestagen-only injectable contraceptives may be associated with a small increase in the risk of CC [25]; it is not clear whether this is related to histology [30]. Progestagen-only injectable contraceptive use was found to be an insignificant risk factor for developing HPV infection and CC.
Schmauz et al. [31] observed a trend of increased risk for CC with increasing number of infections. More recent studies found associations between Chlamydia trachomatis and/or herpes simplex virus-2 and CC risk among HPV-DNA positive women, although the evidence is not uniformly supportive [32]. It has been hypothesized that chronic infection with various sexually transmitted infections may act via inflammation of the cervix leading to genotoxic damage via reactive oxidative metabolites [33]. Our observation of an increased risk of HPV infection and CC among women reporting a history of sexually transmitted diseases or history/existence of warts or history of nonspecific genital infections supports the idea that chronic infection with various sexually transmitted infections may contribute to CC risk.
The most important strength of this study is the use of HPV-DNA positive cases and controls. By comparing HPV-DNA positive cases to HPV-DNA positive controls we were able to examine the associations between different variables and CC, while accounting for any confounding by HPV. This study has several methodological limitations. Studies including the whole spectrum of precancerous lesions as cases are difficult to interpret because the case group includes a mixture of exposed and diseased women, because some have LSIL and some have HSIL/CC. Again, under the premise that HPV is a necessary cause of CC, a more refined case definition could be made on the basis of both HPV and disease statuses. In relation to the control subjects and the selection of HPV exposed women can we assume that HPV-positive control women are carrying a persistent or chronic infection? How long should persistent infection persist to increase the risk of CC: many months or just a few months [27]?
Conclusion
This study provides evidence to support the view that HPV infection and carcinoma of the cervix share many risk factors. Information gathered from this study could be used to give priority to limited screening and treatment services to woman who have specific characteristics that may put them at an increased risk of HPV disease. Additionally, by identifying which women have a higher risk of CC; it may be possible to reduce the number of unnecessary colposcopies.
References
Kjaer SK, Chackerian B, van den Brule AJ, Svare EI, Paull G, Walboomers JM, Schiller JT, Bock JE, Sherman ME, Lowy DR, Meijer CL (2001) High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev 10:101–106
Winer RL, Lee SK, Hughes JP, Kiviat NB, Koutsky LA (2003) Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol 157:218–226
Castle PE, Wacholder S, Lorincz AT, Scott DR, Sherman ME, Glass AG, Rush BB, Schussler JE, Schiffman M (2002) A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J Natl Cancer Inst 94:1406–1414
Lundberg GD (1989) The 1988 Bethesda system for reporting cervical/vaginal cytological diagnosis. JAMA 262:931–934
Coutlee F, Gravitt P, Kornegay J, Hankins C, Richardson H, Lapointe N, Voyer H, Franco E (2002) Use of PGMY primers in L1 consensus PCR improves detection of human papillomavirus DNA in genital samples. J Clin Microbiol 40:902–907
van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJ, Snijders PJ (2002) GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol 40:779–787
de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H (2004) Classification of papillomaviruses. Virology 324:17–27
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19
Munoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348:518–527
Munoz N, Bosch FX, Castellsagué X, Diaz M, de Sanjose S, Hammouda D, Shah KV, Meijer CJ (2004) Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer 111:278–285
Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV (2002) The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 55:244–265
Brinton LA, Tashima KT, Lehman HF, Levine RS, Mallin K, Savitz DA, Stolley PD, Fraumeni JF (1987) Epidemiology of cervical cancer by cell type. Cancer Res 47:1706–1711
Ngelangel C, Munoz N, Bosch FX, Limson GM, Festin MR, Deacon J, Jacobs MV, Santamaria M, Meijer CJ, Walboomers JM (1998) Causes of cervical cancer in the Philippines: a case–control study. J Natl Cancer Inst 90:43–49
Chichareon S, Herrero R, Munoz N, Bosch FX, Jacobs MV, Deacon J, Santamaria M, Chongsuvivatwong V, Meijer CJ, Walboomers JM (1998) Risk factors for cervical cancer in Thailand: a case–control study. J Natl Cancer Inst 90:50–57
Lacey JVJ, Frisch M, Brinton LA, Abbas FM, Barnes WA, Gravitt PE, Greenberg MD, Greene SM, Hadjimichael OC, McGowan L, Mortel R, Schwartz PE, Zaino RJ, Hildesheim A (2001) Associations between smoking and adenocarcinomas and squamous cell carcinomas of the uterine cervix (United States). Cancer Causes Control 12:153–161
Deacon JM, Evans CD, Yule R, Desai M, Binns W, Taylor C, Peto J (2000) Sexual behaviour and smoking as determinants of cervical HPV infection and of CIN3 among those infected: a case-control study nested within the Manchester cohort. Br J Cancer 83:1565–1572
Altekruse SF, Lacey JVJ, Brinton LA, Gravitt PE, Silverberg SG, Barnes WAJ, Greenberg MD, Hadjimichael OC, McGowan L, Mortel R, Schwartz PE, Hildesheim A (2003) Comparison of human papillomavirus genotypes, sexual, and reproductive risk factors of cervical adenocarcinoma and squamous cell carcinoma: Northeastern United States. Am J Obstet Gynecol 188:657–663
Munoz N, Franceschi S, Bosetti C, Moreno V, Smith JS, Shah KV, Meijer CJ, Bosch F (2002) Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case–control study. Lancet 359:1093–1101
Parazzini F, La Vecchia C, Negri E, Fasoli M, Cecchetti G (1988) Risk factors for adenocarcinoma of the cervix: a case–control study. Br J Cancer 57:201–204
Brinton LA, Herrero R, Reeves WC, de Britton RC, Gaitan E, Tenorio F (1993) Risk factors for cervical cancer by histology. Gynecol Oncol 51:301–306
Hildesheim A, Herrero R, Castle P, Wacholder S, Bratti M, Sherman ME, Lorincz AT, Burk RD, Morales J, Rodriguez AC, Helgesen K, Alfaro M, Hutchinson M, Balmaceda I, Greenberg M, Schiffman M (2001) HPV cofactors related to the development of cervical cancer: results from a population-based study in Costa Rica. Br J Cancer 84:1219–1226
Kvale G, Heuch I, Nilssen S (1988) Reproductive factors and risk of cervical cancer by cell type. A prospective study. Br J Cancer 58:820–824
Bjorge T, Kravdal O (1996) Reproductive variables and risk of uterine cervical cancer in Norwegian registry data. Cancer Causes Control 7:351–357
Moreno V, Bosch FX, Munoz N, Meijer CJ, Shah KV, Walboomers JM, Herrero R, Franceschi S (2002) Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case–control study. Lancet 359:1085–1092
Smith JS, Green J, Berrington de Gonzalez A, Appleby P, Peto J, Plummer M, Franceschi S, Beral V (2003) Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet 361:1159–1167
La Vecchia C, Altieri A, Franceschi S, Tavani A (2001) Oral contraceptives and cancer: an update. Drug Saf 24:741–754
Manhart LE, Koutsky LA (2002) Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? A meta-analysis. Sex Transm Dis 29:725–735
Kjaer SK, van den Brule AJ, Bock JE, Poll PA, Engholm G, Sherman ME, Walboomers JM, Meijer CJ (1996) Human papillomavirus—the most significant risk determinant of cervical intraepithelial neoplasia. Int J Cancer 65:601–606
Coker AL, Sanders LC, Bond SM, Gerasimova T, Pirisi L (2001) Hormonal and barrier methods of contraception, oncogenic human papillomaviruses, and cervical squamous intraepithelial lesion development. J Womens Health Gend Based Med 10:441–449
Thomas DB, Ray RM (1995) Depot-medroxyprogesterone acetate (DMPA) and risk of invasive adenocarcinomas and adenosquamous carcinomas of the uterine cervix. WHO collaborative study of neoplasia and steroid contraceptives. Contraception 52:307–312
Schmauz R, Okong P, de Villiers EM et al (1989) Multiple infections in cases of cervical cancer from a high-incidence area in tropical Africa. Int J Cancer 43:805–809
Smith JS, Herrero R, Bosetti C, Munoz N, Bosch FX, Eluf-Neto J, Castellsague X, Meijer CJ, Van den Brule AJ, Franceschi S, Ashley R (2002) Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst 94:1604–1613
Gravitt PE, Castle PE (2001) Chlamydia trachomatis and cervical squamous cell carcinoma. JAMA 285:1703–1704
Acknowledgments
No financial support was received for this paper.
Conflict of interest
The authors declare no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yetimalar, H., Kasap, B., Cukurova, K. et al. Cofactors in human papillomavirus infection and cervical carcinogenesis. Arch Gynecol Obstet 285, 805–810 (2012). https://doi.org/10.1007/s00404-011-2034-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-011-2034-3