Abstract
Objective
This study was performed to evaluate and compare the placental pathology in patients with severe pre-eclampsia (PE) and HELLP syndrome. Moreover, neonatal birth weight was compared between the two groups.
Materials and methods
This cross-sectional prospective study was conducted in Alzahra and Beheshti Hospitals (Isfahan, Iran) between 2007 and 2009. Placentas from 32 patients having severe pre-eclampsia without HELLP (referred to as preeclampsia group) and 25 patients having severe preeclampsia with HELLP syndrome (referred to as HELLP group) were evaluated. The studied parameters included placental weight, chorioamnionitis (either acute or chronic), retroplacental hematoma, placental infarction, intervillous thrombosis, and decidual arteriopathy. Birth weight adjusted for gestational age was also compared between the two groups.
Results
We found statistically more significant frequency of retroplacental hematoma in the PE group compared to the HELLP group (P value 0.00). Despite the relatively high frequency of accelerated villous maturation and decidual arteriopathy in both groups, the difference between the two groups regarding these two parameters was not statistically significant. Other placental features did not show any significant difference between the two groups either. The frequency of small for gestational age births showed no statistically significant difference between the two groups.
Conclusion
Retroplacental hematoma was the only placental pathology that showed statistically significant different frequencies between the two groups. Although this may suggest different underlying pathogenetic mechanisms in these two conditions, further studies are needed to confirm this hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The exact prevalence of preeclampsia is unknown. A prevalence of 26 per 1,000 births has been reported in one study [1–4]. Preeclampsia refers to the onset of hypertension and proteinuria after 20 weeks of gestation in a previously normotensive woman. Its common effect on fetus is intrauterine growth restriction [5, 6].
Some studies report common pathological features in PE including small placentas with decidual arteriopathy, infarcts in central portions, retroplacental hematoma, and intervillous thrombosis [7].
HELLP syndrome refers to a syndrome characterized by microangiopathic hemolysis, elevated liver enzymes, and a low platelet count [8]. It complicates about 20% cases of severe PE. This syndrome probably represents a severe form of preeclampsia, but the relationship remains controversial. As much as 15–20% of the affected patients do not have antecedent hypertension or proteinuria. Coagulopathy is seen in HELLP patients, but it is not a feature of PE [9]. These differences have led some experts to consider HELLP syndrome as a distinct disorder [8, 10, 11].
It is well documented that placenta is the prerequisite for development of HELLP syndrome and preeclampsia. Part of the different clinical manifestations of severe preeclampsia and HELLP syndrome might be explained by different histopathologic characteristics of placentas in these two conditions. The aim of this study was to test this hypothesis by investigating various macroscopic and microscopic features of placenta in pregnancies complicated by preeclampsia or HELLP syndrome.
Materials and methods
This cross-sectional prospective study included 32 pregnant women with severe preeclampsia without HELLP and 25 women with severe preeclampsia further complicated by HELLP syndrome admitted to Alzahra and Beheshti hospitals in Isfahan, Iran between 2007 and 2009. Severe preeclampsia was diagnosed based on the criteria of the American College of Obstetricians and Gynecologists and defined as the presence of a systolic blood pressure ≥160 mm Hg or a diastolic blood pressure ≥110 mm Hg on two occasions 6 h or more apart, proteinuria of 5 g or more in a 24-h period (or 3+ or 4+ persistent proteinuria on urinalysis when 24-h urine evaluation was not available), oliguria (≤500 ml of urine per 24 h), central nervous system or visual disturbances, epigastric pain, pulmonary edema, and significant fetal growth restriction (<10th percentile). Women were characterized as having HELLP syndrome if they had evidence of thrombocytopenia (platelet count < 100,000/μL), evidence of significantly elevated liver enzymes (AST and/or ALT ≥ 70 u/L, which is twice more than the upper limit of normal), and evidence of hemolysis demonstrated by an abnormal peripheral blood smear and elevated lactate dehydrogenase level (>600 u/L) [6]. Women with a history of chronic hypertension, renal disease, cardiac disease, smoking, or diabetes mellitus were excluded from the study.
To determine the coagulation status of the patients, prothrombin time (PT), INR, and partial thromboplastin time (PTT) were measured. INR more than 1 and/or PTT of more than 32 s were considered as coagulopathy.
First trimester ultrasound examination and the reported gestational age based on the biparietal diameter at that time, when available, were used to determine the gestational age at delivery. If ultrasound examination was not performed during the first trimester of pregnancy, the date of LMP and uterine fundal height were used to estimate the gestational age.
A pathologist, blinded to all clinical data except for the gestational age (in order to assess the villous maturation), studied the placental samples. Placentas were submitted to the pathology laboratory in formalin. Formalin-fixed placentas were macroscopically examined to find the macroscopic evidence of any of these pathologies: chorioamnionitis (as evidenced by opacity of membranes), retroplacental hematoma, infarction (recent or old) in more than 5% of placental bulk, the presence of one or more intervillous thrombosis, and other miscellaneous pathologic findings. The umbilical cord was also studied macroscopically to detect any abnormal finding such as knots (either true or false) and cord twisting. The cord and free membranes were then trimmed and the placental disk was weighed. Tissue samples were prepared from each placenta for microscopic assessment as follows: two rolls of the free membranes (chorion and amnion attached to decidua capsularis) and a section of umbilical cord, two full thickness sections from macroscopically normal appearing areas of placenta, and sections from any abnormal macroscopic finding in placenta.
Abruption was diagnosed clinically (acute abruption) and/or histopathologically based on the presence of a retroplacental hematoma. The percentile of placental weight adjusted for gestational age was assigned based on specific placental weight charts.
The representative microscopic slides were then studied to determine the villous maturation according to the gestational age (proportionate, accelerated, or delayed), decidual arteriopathy, infarction, intervillous thrombosis, chorioamnionitis, and retroplacental hematoma. Other miscellaneous findings, if present, were also recorded.
Decidua capsularis attached to the gestational sac was the site examined for the microscopic evidence of decidual arteriopathy. This was available in rolls prepared from free membranes. Decidual arteriopathy was defined as mild if the vessel was ectatic and its wall showed fibrinoid necrosis, and as severe if there was evidence of acute atherosis (accumulation of foamy macrophages and mononuclear cells in the vessel wall).
Chorioamnionitis was defined microscopically as acute when neutrophils were present within the fetal membranes (i.e., amnion and chorion). [12] Chronic chorioamnionitis was diagnosed microscopically when lymphocytic infiltration into the chorionic trophoblast layer or chorioamniotic connective tissue was seen [13] Both acute and chronic chorioamnionitis were included in the study. To consider membrane opacity as the evidence of chorioamnionitis, it was needed to be confirmed by histologic examination.
Data were analyzed by SPSS.11.5 software and ANOVA, Pearson correlation test, Fisher test, Mann–Whitney test, t test and Chi-square test were used for statistical analysis as indicated. P values less than 0.05 were considered as the level of significance.
This study was approved by the ethics committee of Isfahan University of Medical Sciences (Isfahan, Iran) and informed consents were obtained from the women included in the study.
Results
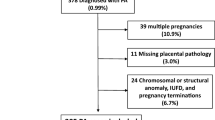
A total of 57 patients enrolled in the study. Of these, 32 (56.1%) had severe preeclampsia (PE) without HELLP and 25 (43.9%) had severe preeclampsia complicated by HELLP syndrome. For convenience, the first group (those having severe preeclampsia without HELLP) and the second one (those with severe preeclampsia complicated by HELLP syndrome) are referred to simply as PE and HELLP groups, respectively. There was no significant difference in baseline characteristics (age, gestational age, number of parities, frequency of small for gestational age neonates, birth weight, and placental weight) between the two groups. Baseline data are presented in Table 1.
We also compared some hematological and biochemical parameters between the pregnant women with PE or HELLP and found significant difference in levels of hemoglobin (Hb) and platelet (Plt), as well as serum lactate dehydrogenase (LDH), uric acid, and creatinine (Cr) between the two groups, while white blood cell count (WBC) had nearly significant difference (Table 2).
Liver function tests (LFT) and coagulation status were compared between the groups. Abnormal LFTs were significantly more frequent in the HELLP group, but there was no significant difference in coagulation status between the two groups (Table 3). Placental weight adjusted for gestational age was compared between the two groups and showed no statistically significant difference (P value 0.13).
In pathologic studies, morphological features of placentas were compared between the two groups. Only one patient in each group had immature placenta (P value 0.68). There was no significant difference between HELLP and pre-eclamptic patients regarding parameters of accelerated villous maturation, decidual arteriopathy, placental infarction, and intervillous thrombosis (P values were 0.15, 0.80, 0.12, and 0.34, respectively) (Fig. 1).
Microscopic features of placental pathologic findings. a Decidual arteriopathy (A1 mild decidual arteriopathy as evidenced by fibrinoid necrosis of the vessel wall with ectasia of the lumen in a specimen from a 33 weeks gestation complicated by severe preeclampsia without HELLP; A2 severe decidual arteriopathy observed as foamy macrophages in the vessel wall in a specimen from a 35 weeks gestation with severe preeclampsia without HELLP) (×400). b Old placental infarction in a 34 weeks gestation complicated by severe preeclampsia with HELLP (×400). c Intervillous thrombosis in a 36 weeks gestation complicated by severe preeclampsia with HELLP (×400)
Although the frequencies of accelerated villous maturation and decidual arteriopathy did not show any statistically significant difference between the two groups, these two pathologic findings showed a relatively high frequency in both groups. Accelerated villous maturation was seen in 31.25% of PE cases and 48% of HELLP patients and decidual arteriopathy was seen in 34.37 and 28% of PE and HELLP cases, respectively. Retroplacental hematoma was significantly more frequent in the PE group (P value 0.00).
No case of chorioamnionitis or stillbirth was seen in either group. We found 2 cases of chorangioma in the HELLP group. This finding was not seen in any cases of PE. However, this finding showed no statistically significant difference between the two groups (P value 0.18). Data concerning placental characteristics are presented in Table 4.
Frequency of accelerated villous maturation, decidual arteriopathy, retroplacental hematoma, placental infarction, and intervillous thrombosis are compared between the two groups and presented as graphs (Figs. 2, 3).
There were nine (28%) neonates small for gestational age (SGA) in the PE group and ten (40%) in the HELLP group. SGA frequency did not show any significant difference between the two groups (P value 0.25) (Table 1). Placental weight adjusted for gestational age was compared between the two groups and showed no statistically significant difference (P value 0.13).
Correlation of placental weight and gestational age was studied. There was evidence of significant correlation between placental weight and gestational age in the PE group (r 0.46, P value 0.00) while this was not the finding in the HELLP group (r 0.20, P value 0.36) (Fig. 4a).
Birth weight was not significantly correlated with the gestational age in the HELLP group. (r −0.04, P value 0.84), while in the PE group birth weight was significantly correlated with the gestational age (r 0.79, P value 0.00) (Fig. 4b).
Birth weight was also compared based on the pathological characteristics of the placenta in either group. In PE patients, mean of the birth weight in the presence of accelerated villous maturation was significantly lower than in those cases with normal villous maturation (1,287.00 ± 419.18 g in accelerated villous maturation versus 2,080.45 ± 563.95 g in normal villous maturation, P value 0.00), while there was no significant difference in the birth weight based on other pathological characteristics. In the HELLP group, there was no significant difference in birth weight based on the pathological features of the placenta.
Discussion
Despite the relatively high frequency of accelerated villous maturation and decidual arteriopathy in both groups, retroplacental hematoma was the only placental pathology that showed statistically significant different frequencies between the two groups. Although this may suggest different underlying pathogenetic mechanisms in these two conditions, further studies are needed to confirm this hypothesis. There was no evidence of significant difference between the two groups for other pathological features.
Since retroplacental hematoma is the histopathologic finding of abruption, more frequency of retroplacental hematoma in PE means that the patients with PE may suffer placental abruption more often than the HELLP group. This finding is consistent with previous studies that introduce hypertensive disorders such as pre-eclampsia as a risk factor of abruption [14–16]. This finding is also in line with Smulian et al.’s study that reported placental abruption as the only differential placental feature of PE and HELLP [17].
Vinnars et al. showed that other than retroplacental hematoma, placental infarction and decidual arteriopathy are also significantly more frequent in preeclampsia [18].
They also found a high frequency of accelerated villous maturation and decidual arteriopathy in both groups. While both studies have some similar findings, differences in other findings could be due to different sample sizes and different racial characteristics.
The present study could not find any difference in the frequency of accelerated villous maturation between the two groups. In this regard, it is noticeable that accelerated villous maturation cannot be adequately evaluated when the placenta is over 37 weeks [18].
Both groups showed positive correlation between placental weight and gestational age, but this correlation was significant only in the PE group. It means that although there is no significant difference in the placental weight between HELLP and PE groups, the placenta grows more rapidly in preeclamptic pregnant women.
On the other hand, no significant correlation of birth weight and gestational age in the HELLP group in comparison to significantly positive correlation in the PE group implies different pathologies leading to different results.
Both the above findings could be related to different general conditions of pregnant women in the HELLP and PE groups. Pregnant women of the HELLP group had significantly more disturbed liver and kidney functions. Therefore by increasing the gestational age, fetal and placental conditions might have been more adversely affected in comparison to the PE group.
Here, there is a question: why do fetuses of the PE and HELLP groups not show any significant differences in birth weight and placental weight? This might be attributable to the more acute nature of HELLP, which leads to a shorter time interval between the diagnosis and delivery and consequently less effect on the final outcome [18].
We found a positive correlation between gestational age and birth weight in the PE group, but this was not the finding in the HELLP group. Perhaps the more acute nature of HELLP and the more critical systemic conditions of the mother do not let the fetus to have enough time and suitable conditions to grow more.
In this study, there was no significant difference in frequency of accelerated villous maturation between the two groups. However, the finding of a reverse relationship between the birth weight of the neonates and accelerated villous maturation in the PE group and the absence of this finding in the HELLP group also confirm the impact of the rapid progression of HELLP on the outcome of pregnancy. It means that fetuses with accelerated villous maturation in the PE group have more time to be affected by decreased uteroplacental blood flow resulting in significantly lower birth weight.
Although some findings of this study concerning the pathological features of placenta in HELLP and PE are different from the results of the previous studies in this field, most of our findings are in favor of the hypothesis that considers PE and HELLP syndrome as separate disorders.
Recent studies at the genetic level have revealed some similarities as well as some differences in placental gene expression between preeclampsia and HELLP. According to the study of Varkonyi et al., placental transcriptomes of early onset preeclampsia and HELLP syndrome overlap. However, gene expression changes may be suggestive of more severe placental pathology in HELLP syndrome compared to preeclampsia [19]. von Steinburg et al. [20] suggest a decrease in matrix remodeling in placentas from HELLP syndrome compared to control pregnancies. Emanuelli et al. have studied the placental expression of transforming growth factor-beta3 (TGF-beta3) in HELLP syndrome and preeclampsia compared to controls. Their results suggest a regulatory role for TGF-beta3 in a variety of cellular events during HELLP syndrome resulting in the dysfunction of maternal–fetal circulation. [21]. Further studies in this field may answer some of the questions regarding the differences between preeclampsia and HELLP.
References
Lindheimer MD, Taler SJ, Cunningham FG (2010) Hypertension in pregnancy. J Am Soc Hypertens 4(2):68–78
Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R (1990) Epidemiology of preeclampsia, eclampsia in the United States, 1979–1986. Am J Obstet Gynecol 163(2):460–465
Centers for Disease Control, Prevention (CDC) (1998) Maternal mortality–United States, 1982–1996. MMWR Morb Mortal Wkly Rep 47(34):705–707
Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D et al (1995) Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal–Fetal Medicine Units. Am J Obstet Gynecol 172(2 Pt 1):642–648
[No authors listed] Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000 183(1):S1–S22
ACOG Committee on Practice Bulletins–Obstetrics (2002) ACOG practice bulletin. Diagnosis, management of preeclampsia, eclampsia. Number 33, January 2002. Obstet Gynecol 99(1):159–167
Goodwin AA, Mercer BM (2005) Does maternal race or ethnicity affect the expression of severe preeclampsia? Am J Obstet Gynecol 193(3 Pt 2):973–978
Sibai BM, Taslimi MM, el-Nazer A, Amon E, Mabie BC, Ryan GM (1986) Maternal-perinatal outcome associated with the syndrome of hemolysis, elevated liver enzymes, low platelets in severe preeclampsia–eclampsia. Am J Obstet Gynecol 155(3):501–509
Curtin WM, Weinstein L (1999) A review of HELLP syndrome. J Perinatol 19(2):138–143
Reubinoff BE, Schenker JG (1991) HELLP syndrome—a syndrome of hemolysis, elevated liver enzymes and low platelet count—complicating preeclampsia–eclampsia. Int J Gynaecol Obstet 36(2):95–102
Sibai BM (1990) The HELLP syndrome (hemolysis, elevated liver enzymes, low platelets): much ado about nothing? Am J Obstet Gynecol 162(2):311–316
Chang KTE (2009) Pathological examination of the placenta: Raison d’être, clinical relevance and medicolegal utility. Singap Med J 50(12):1123–1133
Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V et al (2010) The frequency, clinical significance, pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 23(7):1000–1011
Ananth CV, Savitz DA, Bowes WA Jr, Luther ER (1997) Influence of hypertensive disorders, cigarette smoking on placental abruption, uterine bleeding during pregnancy. Br J Obstet Gynaecol 104(5):572–578
Ananth CV, Savitz DA, Williams MA (1996) Placental abruption, its association with hypertension, prolonged rupture of membranes: a methodologic review, meta-analysis. Obstet Gynecol 88(2):309–318
Ananth CV, Peltier MR, Kinzler WL, Smulian JC, Vintzileos AM (2007) Chronic hypertension and risk of placental abruption: is the association modified by ischemic placental disease? Am J Obstet Gynecol 197(3): 273.e1–273.e7
Smulian J, Shen-Schwarz S, Scorza W, Kinzler W, Vintzileos A (2004) A clinicohistopathologic comparison between HELLP syndrome, severe preeclampsia. J Matern Fetal Neonatal Med 16(5):287–293
Vinnars MT, Wijnaendts LC, Westgren M, Bolte AC, Papadogiannakis N, Nasiell J (2008) Severe preeclampsia with, without HELLP differ with regard to placental pathology. Hypertension 51(5):1295–1299
Várkonyi T, Nagy B, Füle T, Tarca AL, Karászi K, Schönléber J et al (2011) Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar. Placenta 32(Suppl):S21–S29
von Steinburg SP, Krüger A, Fischer T, Mario Schneider KT, Schmitt M (2009) Placental expression of proteases, their inhibitors in patients with HELLP syndrome. Biol Chem 390(11):1199–1204
Emanuelli M, Giannubilo SR, Landi B, Sartini D, Pierella F, Corradetti A et al (2008) Placental overexpression of transforming growth factor-beta3 in the HELLP syndrome. Gynecol Obstet Invest 65(1):1–5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehrabian, F., Mohammadizadeh, F., Moghtaderi, N. et al. Comparison of placental pathology between severe preeclampsia and HELLP syndrome. Arch Gynecol Obstet 285, 175–181 (2012). https://doi.org/10.1007/s00404-011-1948-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-011-1948-0