Abstract
Objective
In our study, we aimed to determine the prevalence of Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum infections among infertile couples and effects of these infections on infertility.
Materials and methods
Prevalence of Chlamydia, Mycoplasma, Ureaplasma antibodies and Chlamydia IgM antibodies and its effect on these agents’ sperm parameters, namely, morphology, density, and motility were investigated among a total of 212 patients including fertile and infertile couples. Chlamydia, Mycoplasma, Ureaplasma antigens were evaluated using ELISA in the cervical and urethral samples. Chlamydia IgM antibody was measured using micro-ELISA in blood samples.
Results
No difference was detected among the fertile and infertile groups in the serological investigation of urethral and cervical samples with respect to the prevalence of Chlamydia, Mycoplasma, Ureaplasma antigens and Chlamydia IgM antibody and sperm parameters (p > 0.05).
Discussion
There is no significant difference between fertile and infertile couples in terms of the prevalence of the above mentioned infections. Accordingly, during the infertility assessment, infertile couples should not be routinely screened for these infective agents without any clinically sound evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infertility has an impact on 10% of the couples in general population [1]. Microbial agents affect fertility by causing obstruction of male and female reproductive systems, pregnancy lost, and significant effects on perinatal mortality and morbidity [2, 3]. Studies showed that Chlamydia, Mycoplasma, and Ureaplasma infections have negative effects on fertility in men and women [4, 5]. Having invasive ability, Chlamydia infection may cause infertility through testicular atrophy, epididymitis, orchitis, and ductal obstruction in men [6–12]. On the other hand, it might cause pelvic inflammatory disease, tubal occlusion, and extra uterine pregnancy in women. 25% of the women diagnosed with tubal infertility are known to have Chlamydia [13, 14].
Due to cervicitis, endomyometritis, salpingitis, pelvic adhesions and tuba ovarian abscess, Mycoplasma and Ureaplasma infections may cause infertility in women. Some studies have revealed that prevalence of antisperm antibodies and accompanying immunological infertility has increased in Ureaplasma antigen positive patients. Furthermore, it has been seen that these infections could cause changes in sperm count, sperm velocity and motility parameters. They have decreased the fertilization capacity of oocyte by reducing sperm capacitation and damaging spermatozoa through increasing free radicals [15–17]. It is known that Chlamydia, Ureaplasma and Mycoplasma infections reduce the success rate of invitro fertilization (IVF) cycles [15, 16].
Materials and methods
In the present study, two groups were formed to investigate the prevalence of Chlamydia, Mycoplasma and Ureaplasma infections and effects of these agents on the sperm parameters of the male partners of the couples using Kruger criteria. 53 fertile couples applied for legal abortion to the family planning clinic constituted the fertile group (n = 112; 53 males, 53 females) while 53 infertile couples attending the infertility clinic for primary and secondary infertility constituted the infertile group (n = 112; 53 males, 53 females). Blood samples and cervical or seminal samples were taken from the patients. Cervical samples were taken from external cervical os using a speculum. Samples from the patients having menstruation were taken after menstruation.
Samples were taken using swabs which were inserted approximately 3–4 cm into the urethral meatus. Then, they were cultured on “Bouillon urée-arginine Lyo” medium and sent to the microbiology laboratory for evaluation. Two different samples were collected: one for Chlamydia and the other for Mycoplasma and Ureaplasma infections.
Collected samples were fed on lyophilized medium (Biomérieux Mycoplasma IST-France) to diagnose Mycoplasma and Ureaplasma infections. Homogenization was maintained followed by slight rotation. Then, 55 μl samples were transferred to microplates containing proliferation wells and antibiotics separately for the quantitative determination of Mycoplasma and Ureaplasma. In an aim to provide anaerobic conditions, 0.1 ml “sterile mineral oil” was added to each well of the microplates. Microplates were closed with lids and incubated at 37°C for 2 days. To further analyze Mycoplasma and Ureaplasma infections, antibiograms were performed using Josamycin, doxycyclin, ofloxacin, erythromycin, tetracycline, and pristinamycin. After 2-day incubation at 37°C, proliferation was evaluated based on the color change. According to the results of antibiogram, antibiotic treatment was given to all the patients who were found to have proliferation. As resistance to doxycycline was not detected in any case, Doxycycline treatment was preferred in patients with positive results. The threshold value of positivity was determined as 1000 ccu/ml for both microorganisms. Any value below this threshold was considered as negative while it was positive for any value above the threshold.
Chlamydial lipopolysaccharide antigen was employed to detect Chlamydia trachomatis antigen in urethral and cervical samples using immunochromatographic method (CHLAMYFAST® Solus. International Microbio, France). 0.9 ml of the RER (Rapid extraction reagent) solution was added to the tube into which a swab containing the sample was placed. Then, it was mixed thoroughly for 10 s and kept at the room temperature for 10–15 min. Extraction liquid was dropped into the round window specific for Chlamydia. The test was done at room temperature in 10–20 min. Blood samples obtained for serologic studies were taken to the laboratory, at the latest, 60 min. Chlamydia IgM antibodies were investigated using Micro-ELISA (Chlamydia trachomatis Ig M ELISA.; Novum Diagnostica GMBH, Germany). Absorbance values 10% above the cut-off were considered positive while values 10% below and above the patient value were regarded as negative.
In an aim to investigate sperm parameters for semen analysis, sperm samples were put into polypropylene tubes after 3 days of sexual abstinence. Then, the ejaculates were allowed to liquefy in the sterilizator (NÜVE®-Jouan, EC 150, France) at 37°C. After liquefaction, samples were evaluated morphologically using Kruger’s strict criteria. Seminal samples were stained (Spermac® Stain.Sperm morphology kit.Ferti-Pro, Belgium). Morphologic defects were classified into four groups namely, head, mid-piece, tail, and acrosome defects. Samples were evaluated twice in the counting chamber (MAKLER®, sefi-medical instruments Ltd.) regarding motility and concentration: once after liquefaction and again after washing (through swim-up technique). Motility was classified into four groups: (a) rapid progressive motility, (b) slow progressive motility, (c) non-progressive motility, and (d) immotility. The eosin-vitality test was done in immotile samples (above 50%). Live and dead cell populations were determined through the evaluation of 100 sperm cells under the light microscope. The criteria for normal semen are as the following: volume above 2.5 ml, sperm concentration above 20 million/ml, normal sperm morphology above 14%, and the sum of rapid (a) and slow (b) progressive motility in two groups as above or below a + b = 50%.
Statistical analyses were carried out with SPSS 11 (Statistical Package for Social Sciences) for Windows. Student t test was used to compare the parametric data between the groups. Nonparametric statistics were performed using Pearson’s Chi Square and Fisher’s Exact Test. An alpha level of 0.05 was established as a criterion for statistical significance.
Results
53 fertile and 53 infertile couples (a total of 212 patients) participated in our study. Infertile couples were selected from those attending infertility clinic. 64.2% (n = 34) of the infertile group had primary infertility while 35.8% (n = 19) of the couples had secondary infertility. When it comes to the fertile group, patients in this group were selected from those attending the family planning clinic with <10-week pregnancy. The mean age was calculated as 26.36 ± 5.77 years for infertile women, 30.43 ± 5.58 years for infertile men, 32.47 ± 5.89 for fertile women, and 37.66 ± 7.02 for fertile men who were considered the control group of the study. One year follow-up of the patients showed 24.52% (n = 13) of pregnancy rate in the infertile group. The mean marriage age of infertile women was found to be 21.06 ± 5.27 while it was 20.66 ± 4.58 for fertile women. No significant difference was detected between two groups in terms of their marriage age, menarche, and age of first sexual intercourse (p > 0.05).
Causes of infertility
Infertile patients participating in our study were followed for 1 year and they were grouped according to the causes of infertility. Table 1 displays the causes of infertility. Male factor infertility seems to be the most common cause of infertility with a percentage of 60.4 (n = 32). Anovulation and PCOS (34%, n = 18), hormonal disorders (32.1%, n = 17), tubal factor (tubal pathologies or intrapelvic adhesions identified during HSG or laparoscopy: 24.5%, n = 13), and unexplained infertility (11.3%, n = 6) were found to be the other causes of infertility, respectively. Two of our patients were diagnosed with endometriosis by laparoscopy.
Urethral samples were collected from 53 fertile and 53 infertile men. Seminal samples were taken from 40 men in the fertile group and 50 men in the infertile group. However, 13 men from the fertile group and 3 men from the infertile group did not want to give seminal samples. Culture materials for bacteriological analysis were taken from all the patients.
The fertile and infertile groups had a mean gravity of 4.08 ± 1.89 and 0.55 ± 0.89, respectively. The mean frequency of sexual intercourse was 2.53 ± 1.31 times per week in the fertile group and 3.55 ± 1.05 times per week in the infertile group. 34% of the women (n = 18) in the infertile group and 28.3% of the women (n = 15) in the fertile group reported smoking. When it comes to males, 34 infertile men (64.2%) and 32 fertile men (60.4%) reported smoking. Smoking habit was not found to be significantly different between the groups (p > 0.05).
Effect of male factor in the infertile group (35.8%, n = 19) was found to be higher than that of the fertile group (7.5%, n = 4) (p < 0.01). Varicocele was the most common cause of male factor infertility with a percentage of 17 in infertile group (n = 9). Six of these nine patients had undergone operation for varicocele.
Sperm disorders
When the disorders in the sperm parameters of the men in both groups were evaluated using Kruger’s strict criteria (Table 2), sperm volume of the men in the infertile group (2.57 ± 1.07 ml) was found to be lower than that of the fertile group (3.25 ± 2.38 ml) (p < 0.05). Sperm count, liquefaction time, viscosity increase, the sum of motility grades a and b (progressive motility), and normal morphology sperm rate between two groups were not found to be different according to Kruger’s criteria (p > 0.05). The sperm count of infertile group was 77.36 ± 47.45 million/ml while fertile group’s sperm count was 88.90 ± 49.95 million/ml. Although sperm concentration did not differ statistically between two groups in terms of motility and sperm morphology, it was found to be lower in infertile men (p = 0.235).
Serology
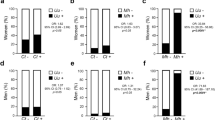
Prevalence of Chlamydia IgM antibodies in infertile and fertile women was positive with the percentages of 7.6 for infertile women and 15.1 for fertile women. 1.9 and 5.7% of these positive values were considered suspicious positive, respectively. On the other hand, antibodies positivity was found in three infertile men (5.7%) and one fertile man (1.9%) (p > 0.05). No infertile men was found to be Chlamydia antigen positive while one man in the fertile group (1.9%) was found to have Chlamydia T antigen. When it comes to women, three women in the infertile group (5.7%) were found to be Chlamydia antigen positive while there was not found to be any antigen in the fertile group (p > 0.05). As seen in Table 3, prevalence of Chlamydia IgM antibodies in infertile couples was not significantly different than that of fertile couples (p > 0.05).
Ureaplasma antigen positivity
Ureaplasma antigen was found to be positive in 11 infertile women (20.8%), 13 infertile men (24.5%), 22 fertile women (41.5%) and 20 fertile men (37.7%), which was not statistically significant (p > 0.05). Sperm volume of Ureaplasma positive men was 2.81 ± 1.07 ml in infertile cases and 4.1 ± 3.71 ml in fertile cases. Sperm volume of ureaplasma negative men was 2.49 ± 1.07 ml in infertile men and 2.74 ± 0.68 ml in fertile men. Sperm volume was not found to differ between Ureaplasma positive and negative men (p > 0.05). Moreover, sperm concentration was not found to be significantly different between ureaplasma negative (77.35 ± 50.93 million/ml) and positive men (77.38 ± 37.97 million/ml) (p > 0.05).
Mycoplasma hominis antigen positivity
Positivity was identified in one female patient in the infertile group (1.9%) and two female patients in the fertile group (3.8%). Mycoplasma infection was found to be positive in two male infertile patients (3.8%) and four male fertile patients. These results were not statistically significant (p > 0.05).
Feeding percentage of Mycoplasma and Ureaplasma in medium was not found to be different between fertile (54.8%, n = 29) and infertile (34%, n = 18) groups (p > 0.05) (Table 4). Antibiograms were also evaluated. Antibiotic resistance was detected in four patients (7.6%) in the infertile group and 12 patients (22.7%) in the fertile group. The most common antibiotic resistance was ofloxacine with a percentage of 11.3.
Antigen positivity for any three antigens was found to be 28.3% in infertile women (n = 15) and 45.3% for fertile women (n = 24). When it comes to males, it was 24.5% in infertile men (n = 13) and 37.7% in fertile men (n = 20). Although there seems to be a difference in both groups in terms of antigen positivity, it was not significantly different (p > 0.05).
As a result of the treatment given to infertile patients, 13 women (24.5%) got pregnant at the 1 year follow-up. Six women (11.3%) got spontaneously pregnant without any treatment while six women (11.3%) got pregnant through IVF. Two of these patients (3.8%) had miscarriage under 20 weeks. 37 patients (69.8%) in the infertile group have not succeeded pregnancy through the treatment or they have been still taking the treatment. Seven patients of the infertility group (13.2%) reported giving up the treatment for any reason.
Discussion
Chlamydia and Ureaplasma infections are among the most common causes of sexual transmitted diseases [18]. As a result of genital duct obstruction and testicular damage, these infections reduce fertility by impairing sperm functions. However, it is quite difficult to diagnose these infections due to their being clinically silent nature, the relative difficulty of the diagnosis, the antibacterial effect of sperm, the high possibility of contamination with other urethral organisms and the difficulty of culturing [18, 19].
Epididymis of the male genital tract has a crucial role on sperm maturation and transport as well as the enhancement of sperm functions. Studies showed that Chlamydia infection is responsible for 40–80% of epididymitis cases [2]. Prevalence of Chlamydia infection was 12.6% among 284 infertile male patients, but no significant differences were noted between Chlamydia positive and negative group in terms of any sperm parameters except sperm volume groups [11].
A study of 1,023 men revealed a relationship between Chlamydia infection and sperm dysfunction and showed significant improvement among these patients after antibiotic treatment [10]. However, in another study, no effect was identified on seminal parameters and sperm penetration tests, but it was stated that Chlamydia infection was a risk factor for tubal factor infertility among infertile couples [20].
A large number of studies have suggested that positive markers for Chlamydia infection are not associated with altered sperm parameters [21–28]. Others, however, have found that Chlamydia infection correlates with reduced sperm motility [6–9], increased proportion of sperm abnormalities [10], significant reductions in semen density, sperm morphology, and viability [29] and increased likelihood of leukocytospermia [9].
In our study, no relationship was observed between Chlamydia infection and sperm parameters. Prevalence of Chlamydia was similar in fertile and infertile groups and no relationship was identified between infertility and Chlamydia occurrence rate. Chlamydia prevalence was found to be between 0.1 and 8.7% among infertile women and tubal infertility risk factor due to pelvic inflammatory disease associated with Chlamydia infection was found to be 10–20% [30, 31]. Our study revealed that the rate of Chlamydia infection among infertile women was 5.7% and that was not different from that of fertile women.
Many studies showed that there was no correlation between serology and antigen detection. The level of bacteria was not associated with serology [31]. In our study, there was not found to be any significant correlation between serology and antigen detection.
According to serologic results, Chlamydia and Ureaplasma infections caught by couples’ male partners are seen in 25% of the cases. The reason behind this is assumed to be the lower concentration of bacteria in seminal fluid, deficiency in the systemic humoral immune response or the possible difficulties encountered during the arrival of immune system cells to the male genital tract [32].
Ureaplasma was mostly determined in cervical secretions and seminal fluids in infertile couples. It was indicated that prevalence of Ureaplasma infection was 20.1% in infertile women and 10–40% in infertile men [33–35]. Ureaplasma prevalence of infertile men (38.7%) was found to be significantly higher than that of fertile men (9.06%) and Ureaplasma adhesions (gold particles) was seen in spilling germ cell surfaces and especially in mid-piece and postacrosomal areas with electron microscopy [36].
A study of artificial Ureaplasma infection in 47 rats revealed a dramatic decrease in spermatogenesis in rat testicles 3–5 weeks after the infection [37]. It was observed that Ureaplasma had negative effects on sperm concentration, morphology and motility. However, these sperm parameters seemed to improve significantly with effective antibiotic treatment [3, 14, 37].
Rodriguez et al. found 47.3% of their infertile population study group to be positive for at least one of the microorganism (with 12.9% being Chlamydia, 0.3% gonococal infection, 23.5% Ureaplasma, 4.8% Mycoplasma and showed that Chlamydia and Ureaplasma were related to infertility [38]. Studies indicated that failed to demonstrate any association between genital Mycoplasma and infertility [15, 39, 40].
In our study, prevalence of Ureaplasma infection was detected in 24.5% of infertile men, 20.8% of infertile women; 37.7% of fertile men and 41.5% of fertile women. No effect of Ureaplasma infection on sperm parameters was found. There was no difference between fertile and infertile groups in terms of the rate of Ureaplasma infection.
Some studies showed that Mycoplasma infection could cause female infertility and impaired sperm parameters. However, in our study, no correlation was found between Mycoplasma infection and sperm parameters and infertility [16, 17, 36].
It was informed that unexplained infertility was seen between 0 and 31% of all infertility cases. The mean percentage of unexplained infertility was found to be 17% [41]. In our study, unexplained infertility percentage was found to be 11.3% (n = 6).
Chlamydia, Ureaplasma and Mycoplasma infections can make some complications in fertile and infertile population. Early diagnosis and treatment are very important to prevent possible complications. These infections may be clinically silent and as a result of this, complication rate may increase. However, in our study, no difference was found between fertile and infertile couples in terms of the effects of these infections on sperm parameters and infertility. Moreover, prevalence of these infections was found to be the same in fertile and infertile groups. During infertility assessment, infertile couples should not be routinely screened for these infections without any clinically sound evidence.
References
Habbema JDF, Collins J, Leridon H, Evers JLH, Lunenfeld B, te Velde ER (2004) Towards less confusing terminology in reproductive medicine: a proposal. Hum Reprod 19:1497–1501
Greendale GA, Haas ST, Holbrook K, Walsh B, Schachter J, Phillips RS (1993) The relationship of Chlamydia trachomatis infection and male infertility. Am J Pub health 83(7):996–1001
Naessens A, Foulon W, Debrucker P, Devroey P, Lauwers S (1986) Recovery of microorganims in semen and relatioship to semen evaluation. Fertil Steril 45:101
Moss TR, Nicholls A, Viercant P, Gregson S, Hawkswell J (1986) Chlamydia trachomatis and infertility. Lancet 2:281
Friberg J (1980) Mycoplasmas and Ureaplasmas in infertility and abortion. Fertil Steril 33:351
Jakiel G, Robak-Cholubek D, Wieczorek P, Bokiniec M (2004) Evaluation of some parameters of human semen with positive chlamydial reaction. Ann Univ Mariae Curie Sklodowska 59:61–64
Gdoura R, Keskes-Ammar L, Bouzid F, Eb F, Hammami A, Orfila J (2001) Chlamydia trachomatis and male infertility in Tunisia. Eur J Contracept Reprod Health Care 6:102–107
Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ (2007) Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril 87:1087–1097
Gallegos G, Ramos B, Santiso R, Goyanes V, Gosalvez J, Fernandez JL (2008) Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil Steril 90(2):328–334
Custo GM, Lauro V, Saitto C, Frongillo RF (1989) Chlamydial infection and male infertility: an epidemiological study. Arch Androl 23:243–248
Cengiz T, Aydoganli L, Baykam M, Mungan NA, Tuncbilek E, Dincer M et al (1997) Chlamydial infections and male infertility. Int Urol Nephrol 29:687–693
Hosseinzadeh S, Eley A, Pacey AA (2004) Semen quality of men with asymptomatic chlamydial infection. J Androl 25:104–109
Gump D, Gibson M, Ashikaga T (1983) Evidence of prior pelvic inflammatory disease and its relationship to Chlamydia trachomatis antibody and intrauterine contraceptive device use in infertile women. Am J Obstet Gynecol 146:153–159
Khron MA, Hillier SL, Nugent NP, Cotch MP, Carey C, Gibbs SR et al (1995) The genital flora of women with intramaniotic infection. J Infect Dis 171:1475–1480
Xu C, Sun GF, Zhu YF, Wang YF (1997) The correletion of Ureaplasma urealyticum infection with infertility. Androl 29:219–226
Styler M, Shapiro SS (1985) Mycoplasma in infertility. Fertil Steril 44:1
Rose BI, Scott B (1994) Sperm motility, morphology, hyperactivation, and ionophore-induced acrosome reactions after overnight incubation with mycoplasmas. Fertil Steril 61(2):341–348
Paavonen J, Wølner-Hanssen P (1989) Chlamydia trachomatis : a major threat to reproduction. Hum Reprod 4:111–124
Purvis K, Chritiansen E (1992) Male infertility; current concepts. Ann Med 24:259
Kruse WE, Gerhard I, Tilgen W (1990) Chlamydial infection a female and/or male infertility factor? Fertil Steril 53:1037
Motrich RD, Cuffini C, Oberti JP, Maccioni M, Rivero VE (2006) Chlamydia trachomatis occurrence and its impact on sperm quality in chronic prostatitis patients. J Infect 53:175–183
Gdoura R, Kchaou W, Ammar-Keskes L, Chakroun N, Sallemi A, Znazen A et al (2007) Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis and Mycoplasma genitalium in semen and first-void urine specimens of asymptomatic male partners of infertile couples. J Androl 29:198–206
De Barbeyrac B, Papaxanthos-Roche A, Mathieu C, Germain C, Brun JL, Gachet M et al (2006) Chlamydia trachomatis in subfertile couples undergoing an in vitro fertilization program: a prospective study. Eur J Obstet Gynecol Reprod Biol 129:46–53
Vigil P, Morales P, Tapia A, Riquelme R, Salgado AM (2002) Chlamydia trachomatis infection in male partners of infertile couples: incidence and sperm function. Andrologia 34:155–161
Ochsendorf FR, Ozdemir K, Rabenau H, Fenner T, Oremek R, Milbradt R et al (1999) Chlamydia trachomatis and male infertility: Chlamydia-IgA antibodies in seminal plasma are C. trachomatis specific and associated with an inflammatory response. J Eur Acad Dermatol Venereol 12:143–152
Habermann B, Krause W (1999) Altered sperm function or sperm antibodies are not associated with chlamydial antibodies in infertile men with leukocytospermia. J Eur Acad Dermatol Venereol 12:25–29
Weidner W, Floren E, Zimmermann O, Thiele D, Ludwig M (1996) Chlamydial antibodies in semen: search for “silent” chlamydial infections in asymptomatic andrological patients. Infection 24:309–313
Eggert-Kruse W, Rohr G, Kunt B, Meyer A, Wondra J, Strowitzki T et al (2003) Prevalence of Chlamydia trachomatis in subfertile couples. Fertil Steril 80:660–663
Land JA, Van Bergen JE, Morré SA, Postma MJ (2010) Epidemiology of Chlamydia trachomatis infection in women and the cost-effectiveness of screening. Hum Reprod Updat 16(2):189–204
Wilkowska-Trojniel M, Zdrodowska-Stefanow B, Ostaszewska-Puchalska I, Zbucka M, Wołczyński S, Grygoruk C et al (2009) Chlamydia trachomatis urogenital infection in women with infertility. Adv Med Sci 54(1):82–85
Dieterle S, Mahony JB, Luinstra KE, Stibbe W (1995) Chlamydial IgG and IgA antibodies in serum and semen are not associated with the presence of Chlamydia trachomatis DNA or r RNA in semen from male partners of infertile couples. Hum Reprod 10:315–319
Byrne GI (1996) Persistant Chlamydial infections : an in vivo reality or a cell culture artifact? Infect Dis obstet Gynecol 4:149–151
Kundsin RB, Driscoll SG, Monson RR, Yeh C, Biano SA, Cochran WD (1984) Association of Ureaplasma urealyticum in the placenta with perinatal morbidity and mortality. N Engl J Med 310(15):941–945
Quinn PA, Shewchuk AB (1983) Serologic evidence of Ureaplasma urealyticum infection in women with spontaneous pregnancy loss. Am J Obstet Gynecol 145:245–250
Naessens A (1988) Serotypes of Ureaplasma urealyticum isolated from normal pregnant woman and patients with pregnancy complications. J Clin Microbiol 26:319–322
Zanchetta R, Busulo F (1985) The effect of Mycoplasma hominis and Ureaplasma urealyticum on hamster egg in vitro penetration by human spermatozoa. Fertil Steril 43:110–114
Keck C, Gerber-Schafer C, Clad A, Wilhelm C, Breckwoldt M (1998) Seminal tract infections: impact on male fertility and treatment options. Hum Reprod Updat 4:891–903
Imudia AN, Detti L, Puscheck EE, Yelian FD, Diamond MP (2008) The prevalence of Ureaplasma urealyticum, Mycoplasma hominis, Chlamydia trachomatis and Neisseria gonorrhoeae infections, and the Rubella status of patients undergoing an initial infertility evaluation. J Assist Reprod Genet 25(1):43–46
Rodriguez R, Hernandez R, Fuster F, Torres A, Prieto P, Alberto J (2001) Genital infection and infertility. Enferm Infec Microbiol Clin 19:261–266
Gump DW, Gibson M, Ashikaga T (1984) Lack of association between genital mycoplasmas and infertility. N Engl J Med 310:937–941
Taylor PJ, Collins JA (2003) Unexplained infertility. Hum Reprod Updat 9:61–76
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Günyeli, İ., Abike, F., Dünder, İ. et al. Chlamydia, Mycoplasma and Ureaplasma infections in infertile couples and effects of these infections on fertility. Arch Gynecol Obstet 283, 379–385 (2011). https://doi.org/10.1007/s00404-010-1726-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1726-4