Abstract
A 63-year-old mother of two, presented with blood-stained vaginal discharge and right sided lower abdominal pain. A MRI examination confirmed a right parametrial mass, abutting the lateral margin of the uterus and the patient had a total abdominal hysterectomy and bilateral salpingo-oophorectomy. Histological examination diagnosed a cotyledonoid leiomyoma, but with a new epithelioid variant. Cotyledonoid leiomyom’s usually have a large, fungating appearance and demonstrate apparent widespread infiltrative growth and extension into the pelvic cavity, broad ligament and retroperitoneal space which may raise significant concern about the possibility of a malignant neoplasm. As these tumours are rare and infrequently encountered, it is imperative that clinicians be aware of this entity as they may pose a significant diagnostic and management challenge when encountered. Awareness of this newly described epithelioid cell variant of cotyledonoid dissecting leiomyoma is necessary for an accurate diagnosis and to facilitate appropriate management decisions at the time of surgery. This new variant further emphasizes the need for meticulous histopathological assessment which should be undertaken to circumvent misdiagnosis. This has direct clinical relevance to all operating gynaecologists and may have implications for litigation because patients may be inappropriately and inadvertently over-treated for an essentially benign condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case report
A 63-year-old woman presented with a blood-stained vaginal discharge and right sided lower abdominal pain. She was Para 2 and had been on hormone replacement therapy for many years after experiencing an early menopause, at age 45. Her past medical history included ischaemic heart disease, angina, hypothyroidism and irritable bowel syndrome. She had given up smoking a few years ago and had an alcohol intake of 10 units (100 ml) per week. Her body mass index was 21.5. On abdominal examination, there was some mild tenderness in the right iliac fossa and bimanual examination revealed a right sided mass.
Ultrasound scan showed a bulky uterus, with a few small, poorly defined myometrial fibroids. The endometrium was well-defined and thin, measuring 2 mm. In the right adnexa there was a vascular, solid mass, which could not be seen separately from the ovary. A magnetic resonance imaging (MRI) examination confirmed a right parametrial mass measuring 4.5 × 3.5 × 3.5 cm, lying alongside the right lateral uterine margin. It also abutted the right ovary, which appeared normal in size. The aetiology of the adnexal mass was not clear, as it did not return the signal characteristics of a non-degenerating fibroid. It was felt that further imaging was unlikely to characterize the abnormality more accurately. As the CA 125 was 17 U/ml (reference range of 0–35 U/ml), the patient was counselled and agreed to have a total abdominal hysterectomy and bilateral salpingo-oophorectomy.
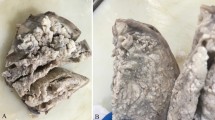
At laparotomy, both ovaries and fallopian tubes appeared normal. The uterus however, was slightly enlarged and there was a lobulated brownish tumour attached to its lateral surface, growing into the right broad ligament in the pattern of cotyledons of placenta (Fig. 1). The right ovary and fallopian tube were seen completely separate from the tumour. The patient made an excellent post-operative recovery and was reviewed after 6 weeks and then 6 months, with no adverse outcomes.
Macro image showing uterus with right broad ligament (seen from postero-lateral aspect) showing tumour growing into the broad ligament. The long arrow shows the fallopian tube and series of short arrows mark the separation of layers of broad ligament by the tumour. Thick arrow shows cut lateral portion of myometrial wall to which the tumour is attached
On macroscopic examination by the pathologist, the uterus measured 9 × 5 × 4 cm. Both the right and left fallopian tubes were unremarkable, measured 4.5 × 0.5 cm each. The ovaries were normal in appearance, with the right measuring 3 × 1 × 0.8 cm and the left 2.5 × 1 × 1 cm, respectively. The lobulated brown tumour attached to the lateral surface of the uterus, measured 4 × 3 × 3 cm. The uterus also contained smaller intramural fibroids and adenomyosis in the uterine myometrium.
Microscopically, the broad ligament tumour was composed of sheets of epithelioid cells with finely granular, pale eosinophilic cytoplasm and central bland nuclei. (Fig. 2) There was no nuclear atypia or mitotic activity. The sheets of tumour cells were interspersed by single and clustered blood vessels with variably thickened walls. (Fig. 3) Many blood vessels were irregular in shape and some were gaping. There was no intravascular tumour. There were focal areas of hyalinisation and myxoid change in the tumour but no necrosis was seen.
The tumour had an intra-uterine component in the right lateral wall, where the sheets of epithelioid tumour cells were seen dissecting between the myometrial smooth muscle bundles (Fig. 4).
Immunohistochemically, the tumour cells stained strongly positive with desmin, vimentin and CD 10. There was also focal but strong positivity with caldesmon and smooth muscle actin. The tumour cells showed heterogenous positivity with P53. CD34 highlighted a diffuse vascular pattern within the tumour and confirmed absence of intravascular tumour. The tumour cells stained negative with CD117, S100, Melanocyte Specific Antigen, Melan A, CAM 5.2, MNF 116, EMA, renal cell carcinoma antigen, calretinin, CK5/6, CD68 and inhibin.
The immunoprofile of this tumour was that of an origin from smooth muscle. Based on the histological appearances and immunohistochemical profile, a diagnosis of cotyledonoid epithelioid dissecting leiomyoma was made.
Discussion
Using an OVID search tool, we searched MEDLINE and EMBASE from 1950 to August 2010 using the keywords: broad ligament tumour, smooth muscle tumour, epithelioid, dissecting, cotelydenoid variant and leiomyoma, we found 33 directly related English language articles.
Leiomyomas are the commonest solid tumours of the broad ligament, but still remain relatively rare. Broad ligament leiomyomas may arise from smooth muscle fibres within the broad ligament or they may originate in adjacent structures and directly extend to involve the broad ligament. [1] In general these tumours tend to be small and are often found incidentally at the time of surgery. However, rather variable sizes of intraligamentous leiomyoma have been reported, with the largest weighing 51 kg. [2] Nonetheless, it is rather rare for broad ligament leiomyoma to enlarge so much as to cause clinical symptoms or complications. But should these occur, broad ligament leiomyoma may cause abdominal pain, a pelvic mass or may even present as an obturator hernia, with a lump in the groin. [3] They may also be a cause for Pseudomeig’s syndrome, presenting with a clinical picture of ascites and pleural effusion, which resolves spontaneously after removal of the underlying tumour. [4] Broad ligament leiomyoma, if complicated will need to be removed surgically. However, laparoscopic management of even large broad ligament leiomyoma is feasible and has been effectively used as an alternative to laparotomy [5].
Uterine leiomyoma may exhibit a variety of different growth patterns, with cotyledonoid dissecting leiomyomata being a rare variant of this. It has previously been described as a “grapelike leiomyoma” and is sometimes referred to as a Sternburg tumour. [6] The current description is derived from the characteristic “presence of numerous deep red, exophytic and bulbous protrusions over the uterus resembling the cotyledons of placenta.” This unusual variant was first reported by Roth et al. [7].
The usual clinical presentation of this tumour variant is that of abnormal uterine bleeding and/or abdominal or pelvic pain. A pelvic mass may also be found. There seems to be no ethnic predilection and it has been reported in woman aged between 23 and 67 years.
Although there have been a number of English language articles directly relating to the subject of cotyledonoid dissecting leiomyoma, there have been only 30 reported cases in the literature so far. One report occurred in a 14 weeks pregnant multipara, who had a laparotomy to excise a large pelvic mass which was causing distension and pain. She subsequently had an elective caesarean section at term without any evidence of residual disease. [8] The other 29 cases occurred in non-pregnant women.
Cotyledonoid dissecting leiomyomata may have a large size, fungating appearance and demonstrate apparent widespread infiltrative growth and extension into the pelvic cavity, broad ligament and retroperitoneal space; which may raise concern about the possibility of a malignant neoplasm. [8] However the histological features of these tumours are of mitotically inactive, bland-looking leiomyoma and clinical outcomes have been uniformly benign. [9, 10] It has been suggested by some authors that frozen section should be done to avoid radical surgery for a potentially benign lesion [11].
It should be noted that several histological variants of cotyledonoid dissecting leiomyoma are described in the literature. These include Cotyledonoid leiomyoma lacking an intramural component, intramural dissecting leiomyoma, which demonstrates intramural dissection but lacks the extrauterine component present in cotyledonoid dissecting leiomyoma [12, 13] and cotyledonoid hydropic intravenous leiomyoma that has the characteristics of cotyledonoid leiomyoma but with an additional intravenous component [14].
Our case is different from all previously reported cases of cotyledonoid dissecting leiomyoma. This is an unusual variant of smooth muscle tumour arising from the lateral uterine myometrium and growing into the broad ligament, displaying cytologically epithelioid and macroscopically cotyledonoid growth patterns. A lack of cellular atypia, mitotic activity and necrosis and no intravascular component suggest that the tumour is likely to behave in a benign fashion.
In all cases so far reported, the tumours were composed of nodules of microscopically recognisable spindle shaped smooth muscle cells arranged in the form of disorganised fascicles. Hydropic change and increased vascularity in perinodular connective tissue was described consistently. On the contrary, this case consisted of sheets of epithelioid cells with finely granular, pale eosinophilic cytoplasm interspersed by clustered and individual, variably sized blood vessels. The smooth muscle origin was recognised only with the help of immunohistochemistry. An intra-operative frozen section is therefore unlikely to help in identifying the smooth muscle origin of this tumour and would in fact, include in the differential diagnoses, a wide range of primary and metastatic lesions such as epithelioid leiomyoma, metastatic carcinoma especially renal cell carcinoma, mesothelioma, melanoma and PEComa.
Whilst cotyledonoid leiomyomas are rare and infrequently encountered, they may create anxiety and pose a significant diagnostic and management challenge for clinicians. Meticulous histopathological assessment should be undertaken in order to circumvent misdiagnosis. [13] Awareness of this newly described epithelioid cell variant of cotyledonoid dissecting leiomyoma is necessary for an accurate diagnosis and facilitation of appropriate management decisions by gynaecologists.
References
Gardner GH, Greene RR, Peckham B (1957) Tumours of the broad ligament. Am J Obstet Gynecol 73:536–555
Muffly T, Vadlamani I, Weed JC (2007) Massive leiomyoma of the broad ligament. Obstet Gynecol 109(2):563–565
Ng Lung Kit HK, Collins RE (1986) Leiomyoma of the broad ligament in an obturator hernia presenting as a lump in the groin. J R Soc Med 79(3):174–175
Brown RS, Marley JL, Cassoni AM (1998) Pseudo-meigs’ syndrome due to broad ligament leiomyoma : a mimic of metastic ovarian carcinoma. Clin oncol 10(3):198–201
Theodoridis TD, Zepiridis L, Grimbizis G et al (2005) Laparoscopic management of broad ligament leiomyoma. J Minim Invasive Gynecol 12(6):469
Brand AH, Scury JP, Planner RS et al (1995) Grapelike leiomyoma of the uterus. Am J Obstet Gynecol 173:959–961
Roth LM, Reed RJ, Sternburg WH (1996) Cotyledonoid dissecting leiomyoma of the uterus: the Sternburg tumor. Am J Surg Pathol 20:1455–1461
Mathew M, Gowri V, Al Hamdani A et al (2007) Cotyledonoid leiomyoma in pregnancy. Am J Obstet Gynecol 109(2):509–511
Gurbuz A, Karateke A, Kabaca C et al (2005) A case of cotyledonoid leiomyoma and review of the literature. Int J Gynecol Cancer 15:1218–1221
Cheuk W, Chan JK, Liu JY (2002) Cotyledonoid leiomyoma: a benign uterine tumor with alarming gross appearance. Arch Pathol Lab Med 126:210–213
Saeed AS, Hanaa B, Faisal AS et al (2006) Cotyledonoid dissecting leiomyoma of the uterus: a case report of a benign uterine tumor with sarcoma like gross appearance and review of literature. Int J Gynecol Pathol 25(3):262–267
Roth LM, Reed RJ (2000) Cotyledonoid leiomyoma of the uterus: report of a case. Int J Gynecol Pathol 19:272–276
Weissferdt A, Maheshwari MB, Downey GP et al (2007) Cotyledonoid dissecting leiomyoma of the uterus: a case report. Diagn Pathol 2:18
Jordan LB, Al-Nafussi A, Beattie G (2002) Cotyledonoid hydropic intravenous leiomyomatosis: a new variant leiomyoma. Histopathology 40:245–252
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soleymani Majd, H., Ismail, L., Desai, S.A. et al. Epithelioid cotyledonoid dissecting leiomyoma: a case report and review of the literature. Arch Gynecol Obstet 283, 771–774 (2011). https://doi.org/10.1007/s00404-010-1716-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1716-6