Abstract
Introduction
The main objective of this study is to illustrate the effectiveness and the safety of standardized technique of laparoscopic lymphadenectomy (LNE), newly introduced in a University Hospital, in patients with gynecologic malignancy.
Materials and methods
A cohort of 104 patients with gynaecologic malignancies (71 with endometrial and 33 with cervical cancer), who underwent laparoscopic pelvic with or without para-aortic LNE between September 2008 and March 2010, were analyzed. Total laparoscopic hysterectomy with bilateral salpingo-oophorectomy (TLH & BSO) was the standard approach for patients with endometrial cancer (n = 71), while laparoscopic (nerve sparing) radical hysterectomy (n = 29), laparoscopic-assisted radical vaginal hysterectomy (n = 2) and radical trachelectomy was the treatment for patients with cervical cancer. All LNE were performed by a learning team under the supervision of an expert surgeon, familiar with the technique.
Results
The median number of pelvic lymph nodes yielded was 22 (range 16–34) and of para-aortic 14 (range 12–24). The mean operative time ± standard deviation for pelvic LNE for each side was 29 ± 17 and 64 ± 29 min for para-aortic LNE. The overall complication rate was 7.6% (n = 8). Two patients were reoperated laparoscopically, one because of postoperative hemorrhage and the other because of lymphocyst formation; laparoconversion was not necessary.
Discussion
Laparoscopic lymphadenectomy performed by a learning team with standardized technique is effective with adequate number of harvested nodes, in acceptable operative time and with low rate of perioperative complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The implementation of laparoscopy in gynecology dramatically changed the surgical management of such patients. Less postoperative pain, shorter hospitalization time, faster recovery, reduced postoperative adhesions and the cost-effectiveness made laparoscopic surgery an attractive operative approach.
The effectiveness and the safety of laparoscopic approach in benign gynecologic diseases have been proven by numerous studies [1, 2]. This is also true for malignant diseases of the female genital tract. However, the introduction of laparoscopy in gynecologic cancer, especially performing lymphadenectomy, demands laparoscopic experience, skillful surgeons, familiarity with the procedure and a standardized technique [3–5]. It is obvious that the management of such patients must fulfill the oncologic standards. It is absolutely mandatory that laparoscopic lymphadenectomy be performed by a team. Each member of the surgical team must be familiar with certain surgical steps. Established procedures improve the perioperative outcomes, the patients’ safety and facilitate the operation [6].
The aim of the present study was to analyze whether laparoscopic pelvic and para-aortic lymphadenectomy in patients with gynecologic cancer can be safely performed by a learning team in a teaching hospital, when it is performed by a standardized technique.
Materials and methods
Data from patients with endometrial and cervical cancer surgically treated in our institution from September 2008 to March 2010, who received laparoscopic pelvic with or without para-aortic lymphadenectomy, were analyzed. Patients’ files, histopathology reports and digital video documentation of the operations were all available at the end of the study. According to the German guidelines, para-aortic lymphadenectomy was performed in patients with endometrial cancer with unfavorable tumor differentiation (grade 3), as well as in patients with cervical cancer with positive pelvic nodes, diagnosed by frozen section. All the patients were treated according standard oncologic protocol: individuals with endometrial cancer by total laparoscopic hysterectomy and bilateral salpingo-oophorectomy (TLH & BSO), while cervical cancer was mainly managed by laparoscopic nerve-sparing radical hysterectomy (LNSRH), by laparoscopic-assisted radical vaginal hysterectomy (LARVH) or radical trachelectomy, with or without BSO. Every patient was informed in detail before surgery and gave written informed consent. Institutional review board approved the study.
Epidemiological characteristics of the patients such as FIGO staging, age, gravity, parity, body mass index (BMI) and uterine weight were analyzed. FIGO staging (International Federation of Obstetrics and Gynecology) for endometrial and cervical cancer was applied [7, 8]. Surgical management and perioperative outcomes were analyzed. Perioperative outcomes such as the operative time of the procedures, the number of yielded nodes, the estimated blood loss (EBL), the decline of hemoglobin (Hb) between preoperative and postoperative levels, the need for blood transfusion and the duration of hospitalization were analyzed. Blood transfusion was considered necessary in case of postoperative Hb levels less than 8 mmol/l. Intraoperative vessels and nerves injury and postoperative complications (lymphocyst, infection, postoperative hemorrhage) were also in the scope of the study.
Surgical technique of lymphadenectomy
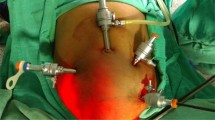
Pelvic and para-aortic lymphadenectomy for endometrial and cervical cancer was performed in an identical manner, with standard technique and under the supervision of a skilled surgeon, familiar with the procedure (A.K.). The learning team consisted of gynecologists with no previous experience in laparoscopic lymphadenectomy and with basic knowledge in gynecologic endoscopy (A.H., M.B., N.C.). The surgeon stood on the left side of the patient, who was placed in Trendelenburg position under general anesthesia. CO2 insufflation via the Verres needle was instituted. A 10-mm trocar was inserted sub-umbilically for the introduction of the camera and intraperitoneal inspection. Four other trocars were placed: one 10 mm diameter in the left upper abdomen and three other 5 mm in the lower abdomen (Fig. 1). The trocar in the left upper abdomen was used as a bowel retractor and for the extraction of the endobag containing the dissected lymph nodes (Fig. 2). Before starting the lymphadenectomy, cul-de-sac lavage for peritoneal cytology was performed in every patient.
For teaching reasons, the pelvic lymphadenectomy procedure was divided in six steps and the para-aortic lymphadenectomy in five steps.
Pelvic lymphadenectomy was defined as the dissection of lymph nodes around the common, external and internal iliac vessels and of the nodes located in the deep obturator fossa. The procedure was always performed using bipolar coagulation in order to prevent bleeding. The caudal border of lymphadenectomy was the profunda circumflex of external iliac vein and artery, the lateral border was the iliopsoas muscle and the cranial was the iliac common artery (bifurcation). The medial border was the lateral umbilical artery, which was identified and isolated.
- Step 1 :
-
The pelvic lymphadenectomy procedure started on the right pelvic side of the patient by opening the peritoneum, starting from the round ligament until the right infundibulopelvic ligament (Fig. 3). Special emphasis was placed on the preservation of the genitofemoral nerve.
- Step 2 :
-
Identification of the ureter was performed at its crossing point with the common iliac artery (Fig. 4).
- Step 3 :
-
Dissection of the lymph nodes along the iliopsoas muscle, starting from the round ligament until the common iliac artery (Fig. 5). Preservation of the genitofemoral nerve was performed with special care.
- Step 4 :
-
Dissection of the lymph nodes along and around the external iliac artery and vein, starting from the profunda circumflex artery and vein up to the common iliac artery (Fig. 6). In this step the dissection of the lymph nodes was always performed by retraction and coagulation of the lymph nodes and lymph vessels from the caudal to the cranial position.
- Step 5 :
-
The obturator fossa was then opened along the external iliac artery and the lateral pelvic wall (iliopsoas muscle). After the identification of the obturator nerve and lateral umbilical artery, en bloc dissection of the lymphatic tissue of the deep obturator fossa and around the internal iliac artery was performed (Fig. 7). Special attention was given for the identification and the preservation of the obturator vessels and nerve.
- Step 6 :
-
Pelvic lymphadenectomy was completed up to the lower part of the para-aortic area removing the nodes around the common iliac artery (Fig. 8).
The procedure used for pelvic lymphadenectomy was similar in patients treated for cervical and endometrial cancer.
Para-aortic lymphadenectomy was divided in right and left para-aortic lymphadenectomy. In cases of patients with endometrial cancer, right para-aortic lymphadenectomy was defined as the dissection of the lymph nodes around the precaval and paracaval area up to the level of the right renal vein. Left para-aortic lymphadenectomy was defined as the dissection of the lymph nodes around the bifurcation (presacral area) and the aorta up to the level of the left renal vein. In cases of patients with cervical cancer, right para-aortic lymphadenectomy was defined as the dissection of the lymph nodes around the precaval and paracaval area up to the level of right ovarian vein, while left para-aortic lymphadenectomy as the dissection of the lymph nodes around the bifurcation and up to the level of inferior mesenteric artery.
The procedure was always performed using bipolar coagulation to prevent bleeding.
- Step 1 :
-
Displacement of the bowel in the upper abdomen; the trocar located in the left upper abdomen was used for bowel’s retractor holding the bowel in the upper abdomen.
- Step 2 :
-
Para-aortic lymphadenectomy started with the incision of peritoneum along the right common iliac artery up to the Treitz ligament. Identification of the right ureter as well as the upper edge of the infundibulopelvic ligament was made (Fig. 9).
- Step 3 :
-
All the lymph nodes around vena cava (precaval and paracaval area) up to the right ovarian vein were dissected (right para-aortic lymphadenectomy) (Fig. 10). Special attention was given to the affluent veins from cava vein to the lymph nodes (Fig. 11).
Fig. 10 - Step 4 :
-
The left para-aortic lymphadenectomy started with the placement of the mesosigmoid in the left upper abdominal cavity and with the identification of the inferior mesenteric artery and the left ureter (Fig. 12).
- Step 5 :
-
Lymph nodes around the bifurcation (presacral area) (Fig. 13) and of the aorta up to the level of inferior mesenteric artery (inframesenteric lymphadenectomy) or up to the level of left renal vein (infrarenal lymphadenectomy) were dissected (Fig. 12).
Bipolar coagulation was used for the dissection of the lymph nodes as well as for hemostasis. During the operation, each patient received antibiotics, while antithrombotic prophylaxis initiated on the day before the surgery and continued until the patient’s discharge. Peritoneum was not closed neither in pelvic nor in para-aortic lymphadenectomy, while two retroperitoneal two drainages were inserted (Douglas and para-aortic space). The drains were removed when the volume of fluid collected was less than 100 ml per day. Postoperatively and after removal of the drains, each patient was routinely controlled for asymptomatic lymphocyst or hydronephrosis by transvaginal and transabdominal ultrasonography, respectively. Operative time was calculated starting from the time of peritoneal incision until the complete removal of the lymph nodes of each respective area.
Statistical analysis
Frequencies, mean values ± standard deviation (SD) and median values with the relative range were calculated using the Statistical Package for Social Science version 17.0 (SPSS Inc, Chicago, IL).
Results
Overall 104 patients were enrolled in the present study, 71 (68%) with endometrioid endometrial cancer (type I) and 33 (32%) with squamous cervical carcinoma. Patients’ FIGO staging is presented in Table 1. The median age of the patients was 57 years ranging between 33 and 74 years. The mean BMI was 26 ± 4.8 kg/m2 and the mean number of previous pregnancies was 2.5 ± 1.1 (Table 1).
All patients with endometrial cancer (n = 71) were surgically treated by total laparoscopic hysterectomy and bilateral salpingo-oophorectomy (TLH & BSO). The patients with cervical cancer were managed by LNSRH type III (n = 29), two patients with radical trachelectomy and two with LARVH type II, with or without BSO. All patients underwent pelvic lymphadenectomy, while 80 patients (77%) were further managed with para-aortic lymphadenectomy. Para-aortic lymphadenectomy was performed in case of grade 3 or intermediate grade 2–3 endometrial cancer and in cases of positive pelvic lymph nodes in frozen section of patients with cervical cancer. Out of 71 patients with endometrial cancer who underwent para-aortic lymphadenectomy, 10 (14%) were found positive, three of them had also positive pelvic nodes, while nοne of the patients with cervical cancer were identified with para-aortic nodal metastasis. Operative data is presented in Table 2.
The overall operative time was 138 ± 36 min. Pelvic lymphadenectomy was performed in 29 ± 17 min for each side, while the mean time for para-aortic lymph nodes dissection was 64 ± 29 min. The median number of yielded pelvic lymph nodes was 22 (range 16–34) and of para-aortic 14 (range 12–24) (Table 3). The median volume of the EBL was 250 ml ranging between 120 and 300 ml. The mean value of the decline of Hb between preoperative and postoperative levels was 1.46 mmol/l. Blood transfusion was necessary in three patients.
One case of injury of the genitor-femoral nerve and four cases of obturator vein injury were noted intraoperatively. Postoperatively, one patient with endometrial cancer was reoperated successfully via laparoscopy because of postoperative hemorrhage (right uterine artery), using bipolar coagulation and endoclips application to control the bleeding. Furthermore, this patient received three blood units. Another patient (cervical cancer) was readmitted because of lymphocyst formation. The complication was managed laparoscopically by a second operation. The overall complication rate (intraoperative and postoperative) was 7.6%, while no conversion to laparotomy was necessary in any of the patients.
Discussion
The present study demonstrated the effectiveness and the safety of laparoscopic pelvic and para-aortic lymphadenectomy in patients with gynecologic cancer, performed by a learning team using standardized technique under the supervision of an experienced operator, as a newly introduced surgical procedure in a University Hospital.
Lymphadenectomy in gynecologic malignancy traditionally was performed by a midline abdominal incision. Querleu et al. and Childers et al., were the pioneers of laparoscopic pelvic lymphadenectomy in patients with cervical cancer, while Netzhat et al. introduced in 1992 laparoscopic para-aortic and pelvic lymphadenectomy [9–11]. Later on, Querleu and Leblanc [12] reported their experience after laparoscopic infrarenal para-aortic lymphadenectomy in ovarian and fallopian cancer. There was no significant difference in the number of removed lymph nodes compared to open surgery [3]. However, others are in disagreement with the previous, showing that only 2/3 of the total number of pelvic nodes can be removed via laparoscopy [14]. Vergote et al. showed almost 10 para-aortic lymphadenectomies are needed to further increase the number of harvested nodes, while others reported a stable nodes number after 20 procedures [5, 15]. The median number of yielded pelvic lymph nodes in our study was 22 (range 16–34), while of para-aortic nodes 14 (range 12–24). Köhler et al. [5] in an analysis of patients with gynecologic malignancy, presented a median number of 19 nodes (range 2–52) performing pelvic lymphadenectomy and 11 nodes (range 1–52) after para-aortic lymphadenectomy, which is comparable to our results. The same authors showed no difference in harvested pelvic nodes during the study period, while there was a significant difference of yielded para-aortic nodes between the early (first 5 years) compared to the late period of the study [5]. The authors concluded that surgical experience contributes significantly to the increasing number of harvested para-aortic nodes during the period of the study.
The mean time for performing para-aortic lymphadenectomy according to our data was 64 ± 29 and 29 ± 17 min for each side of pelvic lymphadenectomy. Kohler et al. [5] demonstrated a similar average of operative time for para-aortic and pelvic lymphadenectomy (64 and 28 min, respectively). However, there is a variation in the duration of lymphadenectomy depending on the extent of the procedure, on how radical it is, on the number of yielded nodes, the experience and the learning curve of the surgical team [4, 6, 16, 17]. Altgassen et al. [6] in a prominent study, underlined the significance of the relationship between the learning curve and the radicality of the procedure, during the improvement of the technique in 108 patients with cervical cancer in a teaching hospital. The authors observed that the operative time for performing the para-aortic lymphadenectomy paradoxically was increased significantly during the late phase of their learning curve (subjects 79–108) compared to early phase (subjects 6–35), (73.2 ± 24.3 vs. 34.8 ± 17.4 min, respectively). The explanation given by the authors was due to the increasing number of harvested nodes during the late phases of the study (late phase, 10.6 ± 4.3 vs. early phase 5.1 ± 4.1). The same observation was not made concerning pelvic lymphadenectomy in which operative time and harvested nodes were similar between the two periods of the study [6].
The overall complication rate of the present series was 7.6%. All the complications were managed laparoscopically and there was no need for laparoconversion during the period of the study. The total rate of postoperative complications was 2.8%, with one case of postoperative hemorrhage. Similar complication rate was shown by others performing laparoscopic pelvic and para-aortic lymphadenectomy [5, 6]. The overall complication rate in Koehler’s study was 8.7%, while the postoperative complication rate was 5.8%, which remained stable during the period of the study. On the contrary, the intraoperative complication rate (2.9%), namely vascular and bowel injury were more often in the first 5 years of the period of the study [5]. However, in the literature the complication rate is different, from null in some reports and up to 10% in others [12–15, 18, 19].
According to the current evidence, laparoconversion related to lymphadenectomy is not very often (~5%) and in some reports there was no need for laparotomy, as we showed [5, 6, 9–11, 13, 19]. However, Scribner et al. [18] in an analysis of 100 cases of gynecologic cancer showed that laparoscopic lymphadenectomy was successfully completed in 71% of the cases. According to the authors the main reasons for conversion to laparotomy were obesity, adhesions and the intraperitoneal disease spread. Furthermore, laparoscopy was successfully completed significantly more often when a gynecologic oncologist was the first assistant compared to community gynecologist (92 vs. 64.5%, respectively) [18].
The small sample size and the lack of evidence related to the follow-up of our patients might be considered as deficiencies of the present clinical trial. However, it must be noted that the main objective of the study was to prove the effectiveness and the safety of laparoscopic lymphadenectomy as a newly introduced technique in a University Hospital, performed by learning surgical team. It is very common in many reports to discus the significance of the learning curve of non-established techniques or newly introduced procedures in teaching hospitals. Some authors consider the first 50 laparoscopic hysterectomies as a cumulative number necessary to standardize the method [20]. Additionally, others demonstrated that the learning experience reached a peak after 75 laparoscopic hysterectomies and this was also true for laparoscopic lymphadenectomies [21], while others believe that the operative time and the complication rate are significantly reduced after the first 40 operations [22]. However, the learning curve is a relevant value. The ability of the learning team to succeed an optimal operative result depends on its previous surgical experience, comprehension of the new procedure and the surgical talent. On the other hand the contribution of an experienced instructor, who introduces the new method with plurality and comprehensiveness, is very crucial for the better understanding of a developing procedure under standardized technique.
In conclusion, we believe that performing pelvic and para-aortic lymphadenectomy under a standard surgical approach, although being a procedure which demands special skills, could be safely performed in adequate operative time and with satisfactory amount of yielded nodes by a learning team and under the supervision of an expert physician, as a newly introduced technique in a teaching hospital.
References
Palomba S, Zupi E, Russo T (2007) A multicenter randomized, controlled study comparing laparoscopic versus minilaparotomic myomectomy: short term outcomes. Fertil Steril 88:942–951
Fanfani F, Fagotti A, Ercoli A (2004) A prospective randomized study of laparoscopy and minilaparotomy in the management of benign adnexal masses. Hum Reprod 19:2367–2371
Mehra G, Weekes ARL, Jacobs IJ, Visvanathan D, Menon U, Jeyarajah AR (2004) Laparoscopic extraperitoneal paraaortic lymphadenectomy: a study of its applications in gynecological malignancies. Gynecol Oncol 93:189–193
Köhler C, Tozzi R, Klemm P, Schneider A (2003) Laparoscopic paraaortic left-sided transperitoneal infrarenal lymphadenectomy in patients with gynecologic malignancies: technique and results. Gynecol Oncol 91:139–148
Köhler C, Klemm P, Schau A (2004) Introduction of transperitoneal lymphadenectomy in a gynecologic oncology center: analysis of 650 laparoscopic pelvic and/or paraaortic transperitoneal lymphadenectomies. Gynecol Oncol 95:52–61
Altgassen C, Possover M, Krause N, Plaul K, Michels W, Schneider A (2000) Establishing a new technique of laparoscopic pelvic and para-aortic lymphadenectomy. Obstet Gynecol 95:348–352
International Federation of Obstetrics, Gynaecology (1989) Annual reports on the results of treatment in gynecologic cancer. Int J Gynaecol Obstet 28:189–193
Creasman W (1995) New gynecologic cancer staging. Gynecol Oncol 58:157–158
Querleu D, Leblanc E, Castelain B (1990) Pelvic lymphadenectomy under celioscopic guidance. J Gynecol Obstet Biol Reprod 19:576–578
Childers JM, Hatch K, Surwit EA (1992) The role of laparoscopic lymphadenectomy in the management of cervical carcinoma. Gynecol Oncol 47:38–43
Netzhat CR, Burell MO, Netzhat FR, Benigno BB, Welander CE (1992) Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol 166:864–865
Querleu D, LeBlanc E (1994) Laparoscopic, infrarenal paraaortic lymph node dissection for restaging of carcinoma of the ovary or fallopian tube. Cancer 73:1467–1471
Possover M, Krause N, Plaul K, Kühne-Heid R, Schneider A (1998) Laparoscopic para-aortic and pelvic lymphadenectomy: experience with 150 patients and review of the literature. Gynecol Oncol 71:19–28
Fowler JM, Carter JR, Carlson JW (1993) Lymph node yield from laparoscopic lymphadenectomy in cervical cancer: a comparative study. Gynecol Oncol 51:187–192
Vergote I, Amant F, Berteloot P, Van Gramberen M (2002) Laparoscopic lower paraaortic staging lymphadenectomy in stage Ib2, II and III cervical cancer. Int J Gynecol Cancer 12:22–26
Sonoda Y, Leblanc E, Querleu D (2003) Prospective evaluation of surgical staging of advanced cervical cancer via a laparoscopic extraperitoneal approach. Gynecol Oncol 91:326–331
Hertel H, Köhler C, Grund D, German Association of Gynecologic Oncologists (AGO) (2006) Radical vaginal trachelectomy (RVT) combined with laparoscopic pelvic lymphadenectomy: prospective multicenter study of 100 patients with early cervical cancer. Gynecol Oncol 103:506–511
Scribner DR, Walker JL, Johnson GA, McMeekin SD, Gold MA, Mannel RS (2001) Laparoscopic, pelvic and paraaortic lymph node dissection: analysis of the first 100 cases. Gynecol Oncol 82:498–503
Spirtos NM, Eisenkop SM, Schlaerth JB, Ballon SC (2002) Laparoscopic radical hysterectomy (type III) with aortic and pelvic lymphadenectomy in patients with stage I cervical cancer: surgical morbidity and intermediate follow-up. Am J Obstet Gynecol 187:340–348
Harkki-Siren P, Sjoberg J (1995) Evaluation and the learning curve of the first one hundred laparoscopic hysterectomies. Acta Obstet Gynecol Scand 74:638–641
Melendez TD, Childers JM, Nour M, Harrigill K, Surwit EA (1997) Laparoscopic staging of endometrial cancer: the learning experience. J Soc Laparoendosc Surg 1:45–49
Childers JM, Hatch KD, Tran AN, Surwit EA (1993) Laparoscopic para-aortic lymphadenectomy in gynecologic malignancies. Obstet Gynecol 82:741–747
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavallaris, A., Kalogiannidis, I., Chalvatzas, N. et al. Standardized technique of laparoscopic pelvic and para-aortic lymphadenectomy in gynecologic cancer optimizes the perioperative outcomes. Arch Gynecol Obstet 283, 1373–1380 (2011). https://doi.org/10.1007/s00404-010-1580-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1580-4