Abstract
Purpose
Based on the reported tocolytic action of the hormone relaxin (RLX) in rodents, locally produced in reproductive tissues and the corpus luteum in mammals, the present study aimed to evaluate the influence of RLX on contraction-mediating cyclooxygenases-1 and -2 (COX) and the contractile prostaglandin PGE2 in human myometrial and decidual cells. Primary cultured cells were obtained from uteri and placentas of term and preterm women undergoing elective caesarean section.
Methods
In vitro culture of primary myometrial and decidual cells, immunocytochemistry, reverse transcription and real-time PCR, Western blot, ELISA.
Results
We demonstrate for the first time an activating effect of RLX for human COX-1 and COX-2 in primary myometrial and decidual cells in vitro.
Conclusions
These effects might potentially contribute to birth-associated induction of contractions in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preterm birth is the main cause for perinatal morbidity and mortality with an incidence up to 12% in the industrial nations [1]. The precise reasons for premature labour and delivery in humans are still unknown [2]. Efficient tocolytics with low risks for mother and foetus and long-term uterine quiescence for more than 7 days are not available to date [3].
The 6-kDa polypeptide hormone relaxin (RLX) belongs to the insulin-like-growth factor family (IGF) and was first described in 1926 by Frederick L. Hisaw [4]. This pregnancy associated peptide is built from a single chain precursor and the major protein consists of 57 amino acids and two polypeptide chains (A and B). One intra- and two interchain disulfide bridges stabilize the structure of the protein in analogy to insulin [5]. Furthermore, according to the maturation of insulin the C chain is removed during processing of the pre-hormone [6]. In 2002, the orphan receptors LGR (leucine-rich G-protein-coupled receptor) 7 and 8 were detected [7], renamed in 2006 to RXFP1 (relaxin family peptide receptors) and 2, respectively [8]. The human genome contains three distinct genes for RLX (H1, H2 and H3) located on chromosome 9 (H1, H2) [9] and chromosome 19 (H3) [10], presumably as a result of evolutionary gene duplications [8]. RLX 1 is expressed locally in human decidua, placenta and prostate [11], while RLX 2, produced in the ovary, is the circulating form [12] and RLX 3 is predominantly expressed in the brain [13]. The expression of RXFP1 on human endometrial and myometrial cells was verified by immunostaining [14]. RXFP2 is in particular expressed on male reproductive tissues (e.g. testis) [15].
Relaxin has numerous effects on the reproductive system including endometrial vascularization and remodelling of connective tissue leading to structural changes, regarding loosening of joints and tendons as well as softening of the cervix in preparation for birth. In pregnant rodents and pigs, RLX also causes uterine relaxation during pregnancy even after OT-stimulated labour [16, 17], while its role in uterine quiescence of higher mammals including humans still remains unclear. However, Weiss et al. [18] reported that elevated serum RLX concentration in pregnant women after ovarian stimulation in the first trimester predicts a risk of preterm labour.
During human pregnancy, RLX is mainly produced systemically by the corpus luteum with the highest serum concentrations (~1 ng/ml) during the first trimester [19, 20]. It is noteworthy that peripherally measured RLX (H2 relaxin) is only produced by the corpus luteum of pregnancy, whereas RLX produced by myometrium, decidua and placenta is likely to act locally.
In myometrium, RLX induces cyclic AMP (cAMP) production by adenylate cyclase (AC) activation. This results in the stimulation of potassium (K+) channels with K+ transport to the extracellular space and activation of protein kinase A (PKA) as well as inhibition of phosphatidylinositol bisphosphate (PIP2) turnover [21, 22] by a specific phospholipase C-β3 (PLC) (Fig. 1) [22–25]. Inhibition of PIP2 turnover leads to diminished release of Ca2+ from the sarcoplasmic reticulum as well as Ca2+ entry from the extracellular space, stabilizing the membrane potential of the cells [26].
Schematic illustration of the RLX signalling cascade in the uterine smooth muscle cell. Arachidonic acid (AA), adenylate cyclase (AC), adenosine-triphosphate (ATP), cyclic adenosine-monophosphate (cAMP), calcium (Ca2+), calcium – calmodulin-complex (CaM), cyclooxygenases (COX), diacylglycerol (DAG), gap junction (GJ), G-protein-coupled receptor (Gp), inositol-triphosphate (IP 3), potassium (K+), myosin-lightchain kinase (MLCK), phosphatidylinositol-4,5-bisphosphate (PIP 2), protein kinase A (PKA), phospholipase C (PLC), prostaglandins (PGs), relaxin (RLX), RLX-receptor 1 (RXFP1), sarcoplasmic reticulum (SR) black arrow stimulation, red arrow inhibition
In rodents, Sanborn et al. [24] showed that RLX was able to stimulate the production of cAMP with subsequent inhibition of PIP2 turnover followed by a reduced production of IP3 and DAG; both second messengers of the OTR signalling cascade promoting uterine relaxation [26].
In summary, RLX is proposed to decrease PIP2 turnover by inhibiting PLC-β3, thus blocking OT action [24]. Although clinical studies in humans could not prove its labour-inhibiting tocolytic effects reported in rodents [27], RLX seems to play a key role in controlling ion contents of muscle cells and therewith, modulating uterine cell contractility.
COX-1 and -2 catalyze the central formation of prostaglandin H2 from AA pivotal for mechanisms of inflammation, tumorogenesis and neurological diseases. Further prostaglandins result from isomerization, mainly supported by synthetases and oxidases. COX-1 and COX-2 reveal a similar structure with 65% identity in their amino acid sequence [28, 29]; an important difference is the amino acid exchange of isoleucine in position 523 to valine resulting in a larger catalytic center in COX-2 and therewith a greater variety of substrates (e. g. for the catalytic conversion of endocannabinoids). COX-1 and -2 are differently regulated and distributed in tissues. A possible induction of COX-1, being for a long time considered as a housekeeping gene, has been reported in tumorigenesis and in normal mouse gestation and preparation for birth [30, 31].
The objective of the present study was to evaluate if RLX might activate COX-1 and COX-2 in cells from human myometrial and decidual cells in vitro, therefore possibly acting similar to IL-1β [32, 33] by down-regulation of the OTR and simultaneously activating the synthesis of PGs.
Materials and methods
Study population and tissue preparation
Parts of the upper margin of the lower uterine segment and decidua were obtained within 20 min of delivery from term (37 + 0 to 41 + 6 weeks of gestation) pregnant women (aged between 22 and 42) (n = 4 different patients) undergoing elective caesarean section before labour.
Informed consent was obtained from all patients in accordance to the admission of the ethics committee of the Heinrich-Heine-University Duesseldorf.
Primary cultures of human myometrium cells (MC) were established as previously described [32]. Briefly, cells were maintained at 37°C in humidified 5% CO2, and medium was changed every other day. Subculturing was performed after short trypsinization [cell-culture agents from Biowest (Nuaillé, France)].
Tissue culture of decidual cells (DC) was modified after Delvin et al. [34]. Briefly, the decidua, separated from foetal membranes, was stored on ice for 20 min in phosphate buffered saline solution (PBS) supplemented with 100 U/ml penicillin/streptomycin and 2.5 μg/ml amphotericin B. Digestion was performed in PBS with 0.2 mg/ml Ca2+, 5 mM Mg2+ and 1 mg/ml collagenase type III (Sigma-Aldrich, Taufkirchen, Germany), 200 μg/ml DNAse type I (Invitrogen, Karlsruhe, Germany) for 4 h at 37°C on an orbital shaker. Digested cells were filtered serially and centrifuged (2.200 rpm, 10 min at 4°C). The cell pellet was resuspended and layered over a discontinuous 10–50% Percoll gradient (Sigma-Aldrich). After centrifugation for 30 min at 3,000 rpm and 4°C, the cells at the 20–30 and 30–40% interface were collected, washed and the cell pellet resuspended in culture medium supplemented with 2 mM glutamine, 1× non-essential amino acids, 1 mM pyruvate, 26 mM NaHCO3, 10% FBS, 100 U/ml penicillin/streptomycin, 2.5 μg/ml amphotericin B and 40 μg/ml gentamycin and plated on gelatinized culture dishes. For subculture, DC were harvested with 0.05% trypsin/0.53 mM EDTA and transferred to culture plates.
Experimental conditions
The culture medium was changed after 24 h and then every other day. All experiments were performed on passages 5–8 at 90% confluence. In all studies, recombinant human RLX H2 (rh RLX) (R&D Systems, Wiesbaden-Nordenstadt, Germany) was used.
The cells were incubated with rh RLX (0–100 ng/ml) for 24 h. Incubations with indomethacin (30 μM), N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (NS-398) (10 μM) and OT (100 nM) (Sigma-Aldrich) were conducted in order to prove the sensitivity of the cells. Cells incubated with medium alone served as control. Medium for incubations of myometrial cells did not contain insulin, which was added to the medium to support cell-growth in the first four passages.
The chosen concentration of RLX refers to Longo et al. [35] and Vogel et al. [36], who used between 10−10 (~0.6 ng/ml) and 10−6 M (~6 μg/ml) for their stimulation protocols.
Immunocytochemistry
The total number of cells were determined by staining with 4,6-diamidino-2-phenylindol (DAPI), the purity of isolated primary myometrial cells by staining with monoclonal mouse anti-smooth muscle α-Actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and for decidua cells with monoclonal mouse anti-vimentin (Dako Cytomation, Hamburg, Germany) [37].
At 90% confluence, myometrial cells were centrifuged and passed on Lab-Tek™ Chamber Slides™ (Nunc, Karlsruhe, Germany). After 3 days of culture, cells were washed, fixed and permeabilized in −20°C methanol for 5 min as described [34]. Briefly, after incubation and washing with PBS, cells were incubated with the fluorescence coupled Alexa® Fluor 488 monoclonal rabbit anti-mouse IgG F(ab′)2 antibody (Molecular Probes, Karlsruhe, Germany, 1:400) for 1 h at room temperature (RT). Cells were then embedded in VECTASHIELD® Mounting Medium with DAPI (1.5 μg/ml) (Vectorlabs, Burlingame, CA, USA) and stored at 4°C to stabilize fluorescence. As a negative control, mouse monoclonal anti-von Willebrand factor (Dako Cytomation) was used.
Decidual cells were treated similarly except for staining procedure [positive staining with mouse anti-vimentin (Dako Cytomation) and negative control using mouse anti-pan cytokeratin (Dako Cytomation) (each 1:5,000)].
For evaluation, a Leica microscope (Leica, DC 300 F) and the Leica IM500 Image Manager program were used.
RNA isolation and reverse transcription-real-time PCR
Total RNA was isolated from myometrial and decidual cells after the single-step method described by Chomczynski and Sacchi [38]. Cells were homogenized according to the manufacturer’s protocol (PEQLAB Biotechnologie GmbH, Erlangen, Germany). Equal amounts of RNA (1.5 μg) were used to perform reverse transcription (RT) and real-time PCR.
TaqMan® primers for human Cox-1 (prostaglandin G/H synthase and cyclooxygenase; Ptgs-1; Hs00377721_m1) and Cox-2 (Ptgs-2; Hs00153133_m1) (Applied Biosystems, Hercules, CA, USA)-cDNAs were used. These primers amplify a 123-bp fragment of the Cox-1 (NM_080591.1) transcript and a 75-bp fragment of the Cox-2 (NM_000963.1) transcript verified by separation of the real-time-PCR products with a 2.5% agarose gel electrophoresis. TaqMan® primers for 18S ribosomal RNA (rRNA) amplifying a 187-bp fragment were used as control.
Prior to reverse transcription, DNA-free RNA was generated by a desoxyribonuclease I (DNase I) (Fermentas, St. Leon-Rot, Germany) digestion (3 U DNase/1.5 μg RNA) as described before [39].
All samples were assayed in duplicate. The linearity of the real-time-PCR products was established as described by Applied Biosystems guidelines for performing relative quantitation of gene expression using the comparative CT method (ΔCT method) (Applied Biosystems, 2004).
Western blot
Protein extracts were prepared from incubated myometrial and decidual cells and resuspended with a 1% SDS-solution. Protein concentration was measured with a Bradford protein assay (Bio-Rad Laboratories).
Proteins (1.5–3 μg) were resuspended in sample loading buffer, heated to 95°C for 10 min and separated by discontinuous SDS (12%)-PAGE. The proteins were transferred electrophoretically to nitrocellulose membranes (Whatman® Schleicher and Schuell, Dassel, Germany). Non-specific binding was blocked by Tris-buffered saline/0.1% Tween®20/5% fat-free dry milk solution for 1 h at RT. For immunodetection, membranes were incubated with mouse anti-human COX-1 (~72 kDa) and COX-2 (~70 kDa) monoclonal IgGs (COX-1 1:100 and COX-2 1:200 dilutions; Santa Cruz Biotechnologies) over night at 4°C. The membranes were washed and incubated with sheep anti-mouse antibodies conjugated to horseradish peroxidase (HRP) (1:500 dilution) for 2 h at RT. Proteins were visualized by enhanced chemiluminescence (ECL Western blotting; Amersham Biosciences, Freiburg, Germany). Protein band sizes were determined using Prestained Protein Molecular Weight Marker (Fermentas). A control sample was included on each blot.
PGE2 ELISA
PGE2 was measured in culture supernatants of myometrial and decidual cells by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instruction (R&D Systems). The minimum detectable PGE2 dose ranged from 8.5–13.9 pg/ml and the inter- and intra-assay coefficients of variation were <11 and <8%, respectively. All cell-culture supernatants were diluted threefold according to the manufacturer’s recommendation. The experiments were performed in duplicate with supernatants of all cells incubated.
Statistical analysis
Different measures of experiments were evaluated by parameters of probability distribution and dependent Student’s t test with equal sample size and variance against media controls. Significance was considered for P < 0.05.
Results
Immunocytochemistry
The isolated myometrial and decidual cells were observed under fluorescence or confocal microscopes to evaluate the purity of the preparation procedure by immunocytochemical staining (Fig. 2). More than 95% of cultured cells were identified as smooth muscle or decidual cells by specific staining with anti-smooth muscle α-actin or anti-vimentin, which displays one of the two major cellular filaments in decidual cells [40], respectively.
Immunostaining of primary myometrial (a–d) and decidual (e, f) cells with either smooth muscle α-actin (c, d) or vimentin (f), DAPI (b, d, f) and fluorescence coupled secondary antibody to evaluate the purity of the cultured cells. a Myometrial cells observed under ×400, phase contrast. b Staining of myometrial cells with DAPI. c Staining of myometrial cells with anti-smooth muscle α-actin and fluorescent secondary antibody. d Merge of B and C to evaluate purity of myometrial cells. e ×200, phase contrast of decidual cells. f Merge of vimentin, DAPI and fluorescence staining of decidual cells under ×400 under a confocal microscope
Figure 2a–d show primary myometrial cells observed with transmitted light (a), the same cells stained with DAPI to visualize the corresponding nuclei under fluorescence (b), the staining with anti-smooth muscle α-actin (c) and the merge of all 3 photos (d). Figure 2e shows a phase contrast of decidual cells and Fig. 2f the merge of vimentin and DAPI under a confocal microscope.
Cox-1 and Cox-2 mRNA analysis
First, primary cultures of human myometrium and decidua (n = 4) were incubated with different RLX concentrations ranging from 0–100 ng/ml for 24 h.
Cox-1 mRNA was activated statistically significant in primary myometrial cells in the samples incubated with 5, 15 and 60 ng/ml RLX (Fig. 3a) compared to the expression of the housekeeping gene 18S ribosomal RNA. Cox-2 mRNA was slightly activated with up to 15 ng/ml RLX, but only statistically significant with 60 ng/ml RLX compared to control (Fig. 3a).
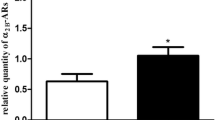
Expression of Cox-1 and -2-mRNA (a, b) and protein (c–f) for primary myometrial and decidual cells incubated for 24 h with different RLX concentrations (0–100 ng/ml) and measurement of PGE2 (g, h) concentration in cell-culture supernatants. a, b Expression of Cox-1 and -2 mRNA normalized with 18S ribosomal RNA shown as mean 2(−ΔΔCT) (*P < 0.05) ± SEM (n = 4 different patients). c–f. Representative western blots for COX-1 (72 kDa) and -2 (70 kDa) protein expression for myometrial and decidual cells. The second lane in the staining for COX-1 results from the similarity of the antigenic epitopes g, h Concentration of contraction-mediating PGE2 in cell-culture supernatants of primary myometrial (g) and decidual cells (h) (n = 4 different patients) isolated at term
For primary decidual cells (Fig. 3b), we detected an activation of Cox-2 mRNA expression using 5 ng/ml RLX and only negligent effects for Cox-1 mRNA.
Western blotting for COX-1 and COX-2
COX-1 and COX-2 protein expression (Fig. 3c–f) were determined after incubation with various RLX concentrations in order to prove the observations on the mRNA level.
As shown in representative blots (Fig. 3c, e), the expression levels of COX-1 and COX-2 in primary myometrial cells corresponded to the activation of mRNA expression (Fig. 3a).
Figure 3d, f show a slight activation of COX-1 using 5–75 ng/ml RLX (Fig. 3d) and a significant increase for 5 ng/ml RLX for COX-2 compared to media control (Fig. 3f).
PGE2 ELISA
As the synthesis of contraction-mediating prostaglandins is a direct consequence of an activation of COX enzymes in human myometrium, we performed a PGE2 ELISA with culture supernatant of primary myometrial (Fig. 3g) and also decidual cells (Fig. 3h) after incubations with different concentrations of RLX. The highest secretion of PGE2 was seen in myometrial cells using 5 ng/ml RLX (Fig. 3g). Concerning the primary decidual cells, we detected a significant peak of PGE2 secretion after incubation with 100 ng/ml (Fig. 3h).
Discussion and conclusions
The multifunctional peptide hormone RLX plays important roles in growth and remodelling during implantation, pregnancy and birth in mammals [41, 42]. So far a growing body of evidence focused on the ability of RLX to inhibit myometrial contractions by activation of cAMP in a phosphoinositide-3-kinase-dependent manner [43].
In the present study, the purpose was to examine RLXs’ effects on COX-1 and -2 in human primary myometrial and decidual cells isolated from term and preterm pregnancies, which was based on RLX’s lacking tocolytic effects in humans [44] shown in clinical studies and being in contrast to its relaxing effect in even OT-stimulated labour in rodents [27]. Upfront we examined RLX’s “relaxing effect” by activating the production of cAMP via stimulation of the adenylate cyclase by a cAMP-ELISA (data not shown). Here we detected a clear contraction-inhibiting effect in human myometrium similar to Kuznetsova et al. [45]. In general, the nonapeptide hormone oxytocin (OT) has several physiological functions involved in parturition and feeding the progeny. The physiologic response to OT is mediated by the oxytocin receptor (OTR), a typical seven transmembrane G-protein-coupled receptor linked to the inositol–triphosphate protein kinase C (IP3–PKC) signal transduction pathway [46]. In myometrium, OT binding to the receptor exerts the ligands dual role in stimulating cell contractility by activating specific phospholipase C (PLC) to produce (1) IP3, leading to the release of intracellular calcium (Ca2+) and (2) diacylglycerol (DAG), stimulating the liberalization of arachidonic acid (AA) with subsequent activation of cyclooxygenases (COX) and production of contractile prostaglandins (PG) [47]. High Ca2+ concentrations contribute to activation of calcium calmodulin (Ca2+CaM) and myosin-lightchain kinase (MLCK) resulting in myometrial contractions. OT expression in human myometrium and decidua obviously increases around the time of parturition [48] as does the expression of the OTR [47]. Gross et al. [49] and Zhao et al. [50] found that the formerly as constitutively expressed known COX-1 is induced on mRNA level, 4–5 days before parturition in mice and therefore shown to be necessary at least for normal termed birth. Based on these findings, we decided to investigate not only the expression of the inducible COX-2, but also COX-1 under the influence of RLX and found a significant activation of Cox-1 and Cox-2 mRNAs in human primary myometrial cells in vitro. We were also able to confirm this activation on protein level showing a concentration-dependent increase in COX-1 and -2 protein. Regarding the production of PGE2, also produced by feto-maternal tissues during parturition, this tends to be inhibited by the non-specific COX-inhibitor indomethacin, whereas there was no obvious inhibition with the COX-2 specific inhibitor NS-398 (data not shown). These results confirm earlier findings of Sparey et al. [51] implicating an important role for COX-1 in birth mechanisms in human myometrium. Due to the incapability to measure transcripts of cyclooxygenases or activity in humans in vivo, we refer to studies in mice reporting an increasing COX-1 transcription and activity in uterus [52] and fetal membranes [53] during late gestation. It was shown that COX-1 activity in the uterine epithelium of mice is the important source of parturition-mediating prostaglandins [30] similar to our results with a concentration-dependent activation. Therefore, we suppose that most of the activation of PGE2 secretion in primary myometrial cells is mediated by COX-1.
COX-1−/− female mice experienced parturition failure, while addition of contractile prostaglandins reduced duration of gestation, sometimes exceeding 21 days in COX-1−/− mice, and improved pup survival [30]. At last, our first results of incubation of primary decidual cells with RLX in vitro provide evidence that there might also be an activating and concentration-depending cross-talk between maternal tissues enforcing parturition-mediating processes as COX-2 could be activated significantly in decidual cells using 5 ng/ml RLX in vitro, too. Furthermore, we could detect a significant increase in PGE2 release using 100 ng/ml RLX in decidual cells.
In previous studies of our group, we could show that RLX down regulates OTR mRNA- and protein-expression favouring uterine relaxation [33].
The results of the present study now seem to explain why there still has been a lack of tocolytic effects in human clinical studies [44]. This might be based on RLX’s effect on COX-1 and -2 activation at the same time. Based upon the down-regulation of OTR by RLX there might be an increase in unbound OT leading to an activation of mitogen-activated protein kinase (MAPK) via NF-κB resulting in a stimulation of COX and prostaglandin production [54, 55]. In former studies concerning the influence of IL-1β in myometrial and decidual cells in vitro, we detected a possible hint for the lack of effects of oxytocin antagonists in the setting of infection-mediated labour [32, 56, 57]; IL-1β increased the expression of IL-6 and OT while reducing the expression of the OTR.
The present study for the first time shows a stimulatory effect of RLX on COX-1 and -2 expression on mRNA- and protein level with subsequent production of contractile PGE2 while earlier results could show an inhibitory effect of RLX on OTR-expression and herewith on the stimulation of contractions [58]. This explains why RLXs’ tocolytic effects seem to be limited to rodents and shows once more the difference between animal models and human physiology [59].
Our results also reveal the complexity of birth mechanisms on cellular basis and give new insights into the regulation of contraction and relaxation of the human uterus yet helping to develop new tocolytic drugs more successful to prevent preterm contractions and therewith decreasing the risks of mother and foetus accompanying preterm birth.
References
WHO The world health report 2000
Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M (2006) The preterm parturition syndrome. BJOG 113(Suppl 3):17–42. doi:10.1111/j.1471-0528.2006.01120.x
Simhan HN, Caritis SN (2007) Prevention of preterm delivery. N Engl J Med 357:477–487. doi:10.1056/NEJMra050435
Hisaw HL (1926) Experimental relaxation of the pubic ligament of guinea pig. Proc Soc Exp Biol Med 23:661–663
Bani D (1997) Relaxin: a pleiotropic hormone. Gen Pharmacol 28:13–22. doi:10.1016/S0306-3623(96)00171-1
Wilkinson TN, Speed TP, Tregear GW, Bathgate RAD (2005) Evolution of the relaxin-like peptide family. BMC Evol Biol 5:14–30. doi:10.1186/1471-2148-5-14
Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ (2002) Activation of orphan receptors by the hormone relaxin. Science 295:671–674. doi:10.1126/science.1065654
Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ (2006) International Union of Pharmacology LVII: recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol Rev 58:7–31. doi:10.1124/pr.58.1.9
Crawford RJ, Hudson P, Shine J, Niall HD, Eddy RL, Shows TB (1984) Two human relaxin genes are on chromosome 9. EMBO J 3:2341–2345
Bathgate RAD, Samuel CS, Burazin TCD, Layfield S, Claasz AA, Reytomas IGT (2002) Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene—novel members of the relaxin peptide family. J Biol Chem 277:1148–1157. doi:10.1074/jbc.M107882200
Bullesbach EE, Schwabe C (2000) The relaxin receptor-binding site geometry suggests a novel gripping mode of interaction. J Biol Chem 275:35276–35280. doi:10.1074/jbc.M005728200
Hansell DJ, Bryant-Greenwood GD, Greenwood FC (1991) Expression of the human relaxin H1 gene in the deciduas, trophoblast, and prostate. J Clin Endocrinol Metab 72:899–904
Liu C, Eriste E, Sutton S, Chen J, Roland B, Kuei C, Farmer N, Jörnvall H, Sillard R, Lovenberg TW (2003) Identification of relaxin-3/INSL7 as an endogenous ligand for the orphan G-protein-coupled receptor GPCR135. J Biol Chem 278:50754–50764. doi:10.1074/jbc.M308995200
Ivell R, Einspanier A (2002) Relaxin peptides are new global players. Trends Endocrinol Metab 13:343–348. doi:10.1016/S1043-2760(02)00664-1
Scott DJ, Fu P, Shen P-J, Gundlach A, Layfield S, Riesewijk A, Tregear GW, Bathgate RAD (2005) Characterisation of the rat INSL3 receptor. Ann N Y Acad Sci 1041:13–16. doi:10.1196/annals.1282.003
Osa T, Inoue H, Okabe K (1991) Effects of porcine relaxin on contraction, membrane response and cyclicAMP content in rat myometrium in comparison with the effects of isoprenaline and forskolin. Br J Pharmacol 104:950–960
Downing SJ, Hollingsworth M (1993) Action of relaxin on uterine contractions. J Reprod Fertil 99:275–282. doi:10.1530/jrf.0.0990275
Weiss G, Goldsmith LT, Sachdev R, Von Hagen S, Lederer K (1993) Elevated first-trimester serum relaxin concentrations in pregnant women following ovarian stimulation predict prematurity risk and preterm delivery. Obstet Gynecol 82:821–828
Bell RJ, Eddie LW, Lester AR, Wood EC, Johnston PD, Niall HD (1988) Antenatal serum levels of relaxin in patients having preterm labour. Br J Obstet Gynaecol 95:1264–1267
Eddie LW, Bell RJ, Lester A, Geier M, Bennett G, Johnston PD, Niall HD (1986) Radioimmunoassay of relaxin in pregnancy with an analogue of human relaxin. Lancet 1:1344–1346. doi:10.1016/S0140-6736(86)91662-4
Anwer K, Hovington JA, Sanborn BM (1989) Antagonism of contractants and relaxants at the level of intracellular calcium and phosphoinositide turnover in the rat uterus. Endocrinology 124:2995–3002
Sanborn BM (2001) Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. Exp Physiol 86:223–237. doi:10.1113/eph8602179
Barata H, Thompson M, Zielinska W, Han YS, Mantilla CB, Prakash YS et al (2004) The role of cyclic-ADP-ribose-signaling pathway in oxytocin induced Ca2+ transients in human myometrium cells. Endocrinology 145:881–889. doi:10.1210/en.2003-0774
Sanborn BM, Qian A, Ku CY, Wen Y, Anwer K, Monga M (1995) Mechanisms regulating oxytocin receptor coupling to phospholipase C in rat and human myometrium. Adv Exp Med Biol 395:469–479
Zhong M, Ku CY, Sanborn BM (2005) Pathways used by relaxin to regulate myometrial phospholipase C. Ann NY Acad Sci 1041:300–304. doi:10.1196/annals.1282.045
Hsu CJ, Sanborn BM (1986) Relaxin treatment alters the kinetic properties of myosin light chain kinetic properties of MLCK activity in rat myometrial cells in culture. Endocrinology 118:499–505
MacLennan AH, Grant P, Bryant-Greenwood G (1995) H-RLX-1 in vitro response of human and pig myometrium. J Reprod Med 40(10):703–706
Garavito RM, DeWitt DL (1999) The cyclooxygenase isoforms: structural insights into the conversion of arachidonic acid to prostaglandins. Biochim Biophys Acta 1441(2–3):278–287
Fitzpatrick FA (2004) Cyclooxygenase enzymes: regulation and function. Curr Pharm Des 10(6):577–588. doi:10.2174/1381612043453144
Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK (2000) Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci USA 97(17):9759–9764. doi:10.1073/pnas.97.17.9759
Rioux N, Castonguay A (2000) The induction of cyclooxygenase-1 by a tobacco carcinogen in U937 human macrophages is correlated to the activation of NF-kappaB. Carcinogenesis 21(9):1745–1751. doi:10.1093/carcin/21.9.1745
Rauk PN, Friebe-Hoffmann U (2000) Interleukin-1β down-regulates the oxytocin receptor in cultured uterine smooth muscle cells. Am J Reprod Immunol 43:85–91. doi:10.1111/j.8755-8920.2000.430204.x
Friebe-Hoffmann U, Baston DM, Chiao JP, Winebrenner LD, Krüssel JS, Hoffmann TK, Hirchenhain J, Rauk PN (2007) The effect of relaxin on the oxytocin receptor in human uterine smooth muscle cells. Regul Pept 138:74–81. doi:10.1016/j.regpep.2006.08.004
Delvin EE, Arabian A, Glorieux FH, Mamer OA (1985) In vitro metabolism of 25-hydroxy-cholecalciferol by isolated cells from human decidua. J Clin Endocrinol Metab 60(5):880–885
Longo M, Jain V, Vedernikov YP, Garfield RE, Saade GR (2003) Effects of recombinant human relaxin on pregnant rat uterine artery and myometrium in vitro. Am J Obstet Gynecol 188(6):1468–1474. doi:10.1067/mob.2003.454 (discussion 1474–1476)
Vogel I, Grønbaek H, Uldbjerg N, Forman A (2004) The influence of amphotericin B and neomycin on the effect of human relaxin-2 on foetal membranes and isolated myometrium. Basic Clin Pharmacol Toxicol 94(3):144–150
Driver PM, Kilby MD, Walker EA, Hewison M, Stewart PM (2001) Expression of 11β-hydroxysteroid dehydrogenase isozymes and corticosteroid hormone receptors in primary cultures of human trophoblast and placental bed biopsies. Mol Hum Reprod 7:357–363. doi:10.1093/molehr/7.4.357
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thio-cyanate–phenol–chloroform extraction. Anal Biochem 162(1):156–159. doi:10.1016/0003-2697(87)90021-2
Kienzle N, Young D, Zehntner S, Bushell G, Sculley TB (1996) DNase I treatment is a prerequisite for the amplification of cDNA from episomal-based genes. Biotechniques 20(4):612–616
Can A, Tekelioglu M, Baltaci A (1995) Expression of desmin and vimentin intermediate filaments in human-decidual cells during first trimester pregnancy. Placenta 16:261–275. doi:10.1016/0143-4004(95)90113-2
Goldsmith LT, Weiss G, Steinetz BG (1995) Relaxin and its role in pregnancy. Endocrinol Metab Clin North Am 24:171–186
Telgmann G, Gellersen B (1998) Marker genes of decidualization: activation of the decidual prolactin gene. Hum Reprod Update 4:472–479. doi:10.1093/humupd/4.5.472
Nguyen BT, Yang L, Sanborn BM, Dessauer CW (2003) Phosphoinositide 3-kinase activity is required for biphasic stimulation of cyclic adenosine 3′,5′-monophosphate by relaxin. Mol Endocrinol 17(6):1075–1084. doi:10.1210/me.2002-0284
Keirse MJ (2003) The history of tocolysis. BJOG 110(Suppl 20):94–97
Kuznetsova LA, Fedin AN, Chistiakova OV, Plesneva SA, Shpakov AO, Pertseva MN (2006) Involvement of adenylyn cyclase signaling mechanisms in relaxing effect of relaxin and insulin on the rat uterus, trachea and human myometrium. Ross Fiziol Zh Im I M Sechenova 92(7):863–871
Phaneuf S, Europe-Finner GN, Carrasco MP, Hamilton CH, López Bernal A (1995) Oxytocin signalling in human myometrium. Adv Exp Med Biol, pp 453–467
Fuchs AR, Fuchs F, Husslein P (1982) Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science 215:1396–1398. doi:10.1126/science.6278592
Chibbar R, Miller FD, Mitchell BF (1993) Synthesis of oxytocin in amnion, chorion and decidua may influence the timing of human parturition. J Clin Invest 91:185–192. doi:10.1172/JCI116169
Gross GA, Imamura T, Luedke C, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ (1998) Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci USA 95(20):11875–11879. doi:10.1073/pnas.95.20.11875
Zhao B, Koon D, Curtis AL, Soper J, Bethin KE (2007) Identification of 9 uterine genes that are regulated during mouse pregnancy and exhibit abnormal levels in the cyclooxygenase-1 knockout mouse. Reprod Biol Endocrinol 5:28. doi:10.1186/1477-7827-5-28
Sparey C, Robson SC, Bailey J, Lyall F, Europe-Finner GN (1999) The differential expression of myometrial connexin-43, cyclooxygenase-1 and -2, and Gsa proteins in the upper and lower segments of the human uterus during pregnancy and labor. J Clin Endocrinol Metab 1999(84):1705–1710. doi:10.1210/jc.84.5.1705
Winchester SK, Imamura T, Gross GA, Muglia LM, Vogt SK, Wright J, Watanabe K, Tai HH, Muglia LJ (2002) Coordinate regulation of prostaglandin metabolism for induction of parturition in mice. Endocrinology 143(7):2593–2598. doi:10.1210/en.143.7.2593
Gupta DK, Sato TA, Keelan JA, Marvin KW, Mitchell MD (2001) Expression of prostaglandin H synthase-1 and -2 in murine intrauterine and gestational tissues from mid pregnancy until term. Prostaglandins Other Lipid Mediat 66(1):17–25. doi:10.1016/S0090-6980(01)00122-8
Molnár M, Rigó J, Romero R, Hertelendy F (1999) Oxytocin activates mitogen-activated protein kinase and up-regulates cyclooxygenase-2 and prostaglandin production in human myometrial cells. Am J Obstet Gynecol 181:42–49. doi:10.1016/S0002-9378(99)70434-5
Belt AR, Baldassare JJ, Molnár M, Romero R, Hertelendy F (1999) The nuclear factor NF-κB mediates interleukin-1β-induced expression of cycloxygenase-2 in human myometrial cells. Am J Obstet Gynecol 181:359–366. doi:10.1016/S0002-9378(99)70562-4
Rauk PN, Chiao JP (2000) Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol 43(3):152–159. doi:10.1111/j.8755-8920.2000.430304.x
Friebe-Hoffmann U, Chiao JP, Rauk PN (2001) Effect of IL-1beta and IL-6 on oxytocin secretion in human uterine smooth muscle cells. Am J Reprod Immunol 46(3):226–231. doi:10.1034/j.1600-0897.2001.d01-6.x
Friebe-Hoffmann U, Baston DM, Hoffmann TK, Chiao JP, Rauk PN (2007) The influence of interleukin-1beta on oxytocin signalling in primary cells of human decidua. Regul Pept 142(3):78–85. doi:10.1016/j.regpep.2007.01.012
Macchiarini F, Manz MG, Palucka AK, Shultz LD (2005) Humanized mice: are we there yet? J Exp Med 202:1307–1311. doi:10.1084/jem.20051547
Acknowledgments
We thank the German Research Foundation (DFG Fr 1402/3–1) for financial support.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baston-Büst, D.M., Hess, A.P., Hirchenhain, J. et al. A possible ambivalent role for relaxin in human myometrial and decidual cells in vitro. Arch Gynecol Obstet 280, 961–969 (2009). https://doi.org/10.1007/s00404-009-1046-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-009-1046-8