Abstract
Introduction
Struma ovarii is a rare form of ovarian neoplasm and consists mainly of thyroid tissue. Ascites has been reported in approximately one-third of all the cases. However, the combination of struma ovarii and elevated CA-125 has rarely been reported.
Materials and methods
We described a case of benign struma ovarii, presenting with the clinical features of ovarian cancer: large complex pelvic mass, gross ascites and markedly elevated serum CA-125 levels. Surgical excision of the ovarian mass was followed by rapid resolution of the ascites and reduction of the serum CA-125 level.
Conclusion
Struma ovarii can mimic ovarian malignancy clinically, when presented with ascites and an elevated CA-125 level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Struma ovarii is a rare ovarian tumor composed of thyroid tissue, which is classified as a variant of mature ovarian teratoma [1]. Although 5–37% of these cases undergo malignant transformation, this tumor is generally benign in nature [2]. Most of the patients had asymptomatic mass, and diagnosis was usually made postoperatively by histologic examination. Ascites occurred in one-third of the cases [3]. However, this is rarely accompanied by elevated serum CA-125 level. Although recently some cases of struma ovarii associated with pseudo-Meigs’ syndrome and elevated serum CA-125 levels were reported and the pleural effusion is supposed to be caused when ascites is transported through the diaphragm or lymphatics. Nevertheless, we cannot confirm that they are totally two different symptoms or the pleural effusion is just the result of the progressive ascites, because only a few cases had been reported. On the other hand, though struma ovarii has been reported associated with Graves’ disease [4], struma ovarii in a patient with recurrent non-toxic multinodular goiter has never been described. We here report a patient of struma ovarii having the history of recurrent huge non-toxic goiter, who was initially thought to have an ovarian malignancy.
Case report
A 56-year-old Chinese woman presented with increasing abdominal distension for 2 months. She had menopause at the age of 51. Her family history was unremarkable. The patient did not complain of any pain, urinary or bowel symptoms. She had no symptoms of hyperthyroidism.
The patient had a history of nodular goiter with partial thyroidectomy done in 1986 in Mainland China. At that time, she did not have symptoms of hyperthyroidism and the blood results for thyroid function was unknown. Three years later, she was diagnosed to have recurrent multinodular goiter. There was sudden increase in size in 2002, associated with dysphagia. Thyroid function was normal. Chest X-ray showed deviation of trachea to right side. Computed tomography scan of the neck and thorax found multinodular goiter with displacement of trachea. Total thyroidectomy was then performed and histology confirmed nodular hyperplasia. Thyroxine sodium supplement of 100 μg per day was started postoperatively and the patient became euthyroid.
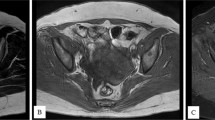
The patient presented to our unit in September 2007 because of abdominal distension, and physical examination revealed marked ascites. Abdomen and pelvic ultrasound revealed gross ascites with a complex solid mass in the left adnexa, about 6.6 × 5.8 × 4.7 cm in size. Computed tomography scan of abdomen and pelvis revealed a fatty mass at left adnexa which was suggestive of a teratoma. There was another irregular soft tissue mass noted closely abuting it, suspicious of malignant change or concomitant ovary tumor (Fig. 1). Paracentesis was performed for symptomatic relieve while awaiting operation which yielded 3,210 ml straw-colored fluid. Cytological examination of the ascitic fluid revealed no malignant cells. Chest X-ray was negative for pleural effusion or lung metastasis. Serum CA-125 level was 5,218 μ/ml (normal value <35 μ/ml). The AFP and CEA levels were within normal range.
Fatty mass at left adnexa suggestive of teratoma. Another irregular soft tissue mass is also noted closely abutting it at POD, suspected malignant change of the teratoma or concomitant ovary tumor. Gross ascites with increase densities over greater omentum. Peritoneal carcinomatosis cannot be excluded
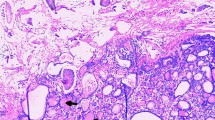
She was taken to the operating room for a laparotomy. The patient was found to have ascites and 5,000 ml of clear fluid was drained upon entrance to the peritoneal cavity. A 6 × 5 × 4 cm vascular, fleshy tumor was found arising from left ovary with contact bleeding. Beneath which noted a 3 cm teratoma consisting of hair and sebaceous material. Uterus and both fallopian tubes were normal. Omentum was inflammatory-looking, but there was no obvious tumor deposits. There was no enlarged lymph nodes or metastatic lesions noted. Left tube and ovary were removed for frozen section and it was suggestive of coexisting mature teratoma and struma ovarii. Total abdominal hysterectomy, right salpingo-oophorectomy and omentectomy were also performed. The final histology revealed left ovarian struma ovarii accompanying a mature cystic teratoma. The tumor tissue is composed of colloid-filled follicles of variable size, which are lined by bland-looking cuboidal cells (Fig. 2). Some have abundant eosinophilic cytoplasm consistent with Hurthle cell changes. In areas, clusters of follicular cells devoid of colloid are present. The stroma is edematous in areas. Immunohistochemically, the cells are diffusely positive for thyroglobulin. The right ovary, fallopian tube, uterus, and omentum were normal, and the ascitic fluid was negative for malignant cells. The patient recovered uneventfully and was discharged on day 7 postoperatively. CA-125 level had reduced to 10.8 μ/ml 4 months after operation. TSH and free T4 were within the normal range. The patient was well with no evidence of recurrent ascites.
Discussion
Struma ovarii is a rare benign tumor of ovary. It usually presents with asymptomatic mass and is diagnosed histologically after surgical resection. However, it has been reported that ascites is present in one-third of the cases [3]. Several hypotheses have been postulated to explain the origin of ascites. It probably occurs by means of a transudative mechanism through the tumor surface, which exceeds the peritoneum’s resorptive capacity. Other possible mechanisms include obstruction of peritoneal lymphatics by tumor or increased permeability of the neovasculature with protein leakage and an inflammatory reaction [5]. Majority of the studies found that ascites disappeared completely after tumor removal. As in our case, the ascites totally subsided after the operation.
Serum tumor markers may be helpful in determining if a mass likely to be malignant. CA-125 is elevated (>35 μ/ml) in over 80% of epithelial ovarian cancers and a smaller proportion of endometrial, bowel, breast, lung and other malignancies. Non-malignant causes of elevated CA-125 can be due to conditions, such as menstruation, pregnancy, endometriosis, infections and ovarian fibroma [6]. When ovarian fibroma associated with pleural effusion and ascites which resolve following excision was referred to Meigs’ syndrome and was first described by Meigs and Cass [7]. When the tumor is other benign ovarian tumor, the condition is called pseudo-Meigs’ syndrome. Meigs’ syndrome with marked elevation of CA-125 is an unusual clinical condition and only reported in 27 cases in the literature. The most likely histopathology is fibroma; others included thecoma, granulose cell tumor and Brenner tumor [8]. The exact mechanism that accounts for the elevation of CA-125 in Meigs’ syndrome is still unknown; however, a possible explanation is the irritation and subsequent inflammation of pleura and peritoneum surface produced by the presence of free fluid in these spaces. The benign primary ovarian tumor by secondary irritation of adjacent mesothelial cells, ascites and pleural effusion may be responsible for the release of or increase in CA-125 production on the surface of these serosal membranes [9].
An ovarian mass associated with ascites and an elevated serum CA-125 level in a postmenopausal woman generally suggests a malignant process. A MEDLINE search of the English language literature found eight case reports of struma ovarii in association with ascites and elevated CA-125 level. Details of those reports were shown in Table 1. All the cases were initially suspected to be a malignant tumor.
Recently, some authors have reported cases of struma ovarii associated with pseudo-Meigs’ syndrome and elevated serum CA-125 levels. We also had a MEDLINE search of the English language literature for struma ovarii associated with pseudo-Meigs’ syndrome and elevated serum CA-125 levels and found eight case reports totally too. Details of those reports were shown in Table 2. Pleural effusion is thought to be caused when ascites is transport through the diaphragm or lymphatics. In those cases with ascites but absence of pleural effusion is thought probably due to the early diagnosis and timely treatment. But from review of the literatures, the amount of ascites seems have not relation to induce pleural effusion. As in our case, the amount of ascites was 8,210 ml, but no pleural effusion was found. Of fibromas, there is 10–15% associated with ascites, but only 1% presents ascites and pleural effusion simultaneously [5]. However, we cannot confirm that they are totally two different syndromes or the pleural effusion is just the result of the progressive ascites, because only a few cases had been reported.
Although struma ovarii contains thyroid tissue, only 5% of the cases have features of hyperthyroidism [1]. All but one of the cases listed in Table 1 had symptoms of hyperthyroidism. The particular case had history of Graves’ disease but it was resistant to the medical treatment and thyroidectomy was then performed [10]. After surgery, the hyperthyroidism persisted. The patient subsequently presented ascites and a large pelvic mass was found. The diagnosis of struma ovarii was finally made after surgical removal. The patient became euthyroid and CA-125 level returned to normal after operation. Although our patient showed no symptoms or clinical signs of hyperthyroidism, interestingly she had a history of recurrent non-toxic goiter which required total thyroidectomy. In the literature, struma ovarii coexisting with history of recurrent huge non-toxic goiter has not been report. Non-toxic nodular goiters are common, even in areas in which iodine intake is sufficient. Both environmental and genetic factors play a role in the pathogenesis [11]. However, in our patient, we cannot exclude that the coexistence struma ovarii may be just a coincidence or it was some intrinsic factor which caused the abnormal growth of thyroid tissue in different part of her body.
In summary, we described a case of struma ovarii presented with marked ascites and elevated CA-125 levels who also had a history of recurrent huge non-toxic goiter. Although similar cases have been reported before, the interesting history and presentation of this case may be of interest to others who encounter similar situation in the future. Furthermore, we reviewed the literatures of the two similar syndromes which only had difference on concurrence of pleural effusion. Though we cannot draw a conclusion on these two interesting condition, we hope that more cases will be reported in future to increase our knowledge on them.
References
Russell P, Anatine P (1989) Monodrama and highly specialized teratomas. Surgical pathology of the ovaries. Churchill Livingstone, Edingburgh, pp 441–444
Rosenblum NG, LiVolsi VA, Edmonds PR, Mikuta JJ (1989) Malignant struma ovarii. Gynecol Oncol 32(2):224–227. doi:10.1016/S0090-8258(89)80037-X
Amr SS, Hassan AA (1994) Struma ovarii with pseudo-Meigs’ syndrome. Eur J Obstet Gynecol Reprod Biol 55:205–208. doi:10.1016/0028-2243(94)90039-6
Mimura Y, Kishida M, Masuyama H (2001) Coexistence of Graves’ disease and struma ovarii: case report and literature review. Endocr J 48:255–260. doi:10.1507/endocrj.48.255
Abad A, Cazorla E, Ruiz F et al (1999) Meigs’ syndrome with elevated CA125: case report and review of the literature. Eur J Obstet Gynecol Reprod Biol 82:97–99. doi:10.1016/S0301-2115(98)00174-2
Leung YC, Hammond IG (1993) Limitations of CA125 in the preoperative evaluation of a pelvic mass: struma ovarii and ascites. Aust N Z J Obstet Gynaecol 33:216–217. doi:10.1111/j.1479-828X.1993.tb02400.x
Meigs JV, Cass JW (1937) Fibroma of the ovary with ascites and hydrothorax, with a report of seven cases. Am J Obstet Gynecol 33:249–267
Morán-Mendoza A, Alvarado-Luna G, Calderillo-Ruiz G, Serrano-Olvera A, López-Graniel CM, Gallardo-Rincón D (2006) Elevated CA125 level associated with Meigs’ syndrome: case report and review of the literature. Int J Gynecol Cancer 16(Suppl 1):315–318. doi:10.1111/j.1525-1438.2006.00228.x
Lin JY, Angel C, Sickel JZ (1992) Meigs syndrome with elevated serum CA125. Obstet Gynecol 80:563–566
Guida M, Mandato VD, Di Spiezio Sardo A et al (2005) Coexistence of Graves’ disease and benign struma ovarii in a patient with marked ascites and elevated CA125 levels. J Endocrinol Invest 28:827–830
Brareman LE, Utiger RD, Werner and Ingbar’s (2004) The thyroid. A fundamental and clinical text, 9th edn. Lippincott Williams & Wilkins, pp 873–885
Jotkowitz MW, Gee DC (1993) Unique case of massive ascites, extreme elevation of serum CA125 tumor marker. Aust N Z J Obstet Gynaecol 33:453–454
Mancuso A, Triolo O, Leonardi I, De Vivo A (2001) Struma ovarii: a rare benign pathology which may erroneously suggest malignancy. Acta Obstet Gynecol Scand 80:1075–1076. doi:10.1034/j.1600-0412.2001.801121.x
Loizzi V, Cappuccini F, Berma ML (2002) An unusual presentation of struma ovarii mimicking a malignant process. Obstet Gynecol 100:1111–1112. doi:10.1016/S0029-7844(02)02154-3
Bokhari A, Rosenfeld GS, Cracchiolo B, Heller DS (2003) Cystic struma ovarii presenting with ascites and an elevated CA125 level. J Reprod Med 48:52–56
Rim SY, Kim SM, Choi HS (2005) Struma ovarii showing clinical characteristics of ovarian malignancy. Int J Gynecol Cancer 15:1156–1159. doi:10.1111/j.1525-1438.2005.00328.x
Mitrou S, Manek S, Kehoe S (2008) Cystic struma ovarii presenting as pseudo-Meigs’ syndrome with elevated CA125 levels. A case report and review of the literature. Int J Gynecol Cancer 18(2):372–375. doi:10.1111/j.1525-1438.2007.00998.x
Paladini D, Vassallo M, Sglavo G, Nappi C (2008) Struma ovarii associated with hyperthyroidism, elevated CA 125 and pseudo-Meigs syndrome may mimic advanced ovarian cancer. Ultrasound Obstet Gynecol 32(2):237–238. doi:10.1002/uog.5399
Obeidat BR, Amarin ZO (2007) Struma ovarii with pseudo-Meigs’ syndrome and elevated CA125 levels. J Obstet Gynaecol 27(1):97–98. doi:10.1080/01443610601076267
Loizzi V, Cormio G, Resta L, Fattizzi N, Vicino M, Selvaggi L (2005) Pseudo-Meigs syndrome and elevated CA125 associated with struma ovarii. Gynecol Oncol 97(1):282–284. doi:10.1016/j.ygyno.2004.12.040
Huh JJ, Montz FJ, Bristow RE (2002) Struma ovarii associated with pseudo-Meigs’ syndrome and elevated serum CA 125. Gynecol Oncol 86(2):231–234. doi:10.1006/gyno.2002.6741
Long CY, Chen YH, Chen SC, Lee JN, Su JH, Hsu SC (2001) Pseudo-Meigs syndrome and elevated levels of tumor markers associated with benign ovarian tumors—two case reports. Kaohsiung J Med Sci 17(11):582–585
Bethune M, Quinn M, Rome R (1996) Struma ovarii presenting as acute pseudo-Meigs syndrome with an elevated CA 125 level. Aust N Z J Obstet Gynaecol 36(3):372–373. doi:10.1111/j.1479-828X.1996.tb02734.x
Acknowledgments
The authors would like to thank Professor Annie Cheung for her support on pathological field.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mui, M.P., Tam, Kf., Tam, F.K.Y. et al. Coexistence of struma ovarii with marked ascites and elevated CA-125 levels: case report and literature review. Arch Gynecol Obstet 279, 753–757 (2009). https://doi.org/10.1007/s00404-008-0794-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-008-0794-1