Abstract
Prolidase is a specific imidodipeptidase involved in collagen degradation. The increase in the enzyme activity is believed to be correlated with the increased intensity of collagen degradation. The aim of this study was to evaluate the serum prolidase activity and its relationship between bone turnover markers and bone mineral density (BMD) in postmenopausal osteoporosis. The study included 45 postmenopausal osteoporotic, 55 postmenopausal nonosteoporotic and 38 premenopausal healthy women. BMD was measured at the femoral neck and lumbar spine with DEXA. T score was more than 2.5 SD below the normal at the lumbar spine or femoral neck in postmenopausal osteoporotic patients. Serum levels of prolidase, C-terminal telopeptide of type I collagen (C-telopeptide), total alkaline phosphatase (ALP), osteocalcin (OC), urinary deoxypyridinoline (Dpd) and urinary creatinine were also assayed. C-telopeptide, total ALP, OC, urinary Dpd levels were significantly higher in postmenopausal osteoporotic group compared with premenopausal women. However, there was no statistical difference in serum prolidase activity between the three groups. There were also no significant correlations between serum prolidase and any biomarkers of bone turnover as well as BMD. To conclude, in postmenopausal osteoporotic women with increased bone turnover, serum prolidase concentration was not correlated with the biomarkers of bone formation or bone resorption and with BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a serious public health problem that is estimated to affect up to one-third of the postmenopausal women [2]. It is characterized by low bone mass, microarchitectural deterioration of bone tissue, caused by an imbalance of skeletal turnover maintained by two opposite but normally balanced processes of bone formation and resorption that leads to bone fragility and a consequent susceptibility to fracture [4]. Indeed, type I collagen constitutes 90% of the organic bone matrix and the turnover of the organic matrix also increases in postmenopausal osteoporosis. The increased rate of collagen synthesis may also lead to a change in the quality of collagen fibres. Oxlund et al. [13] reported that bone collagen from cancellous vertebral bone taken from deceased individuals with osteoporosis had increased extractability and a substantial reduction in the concentration of divalent reducible collagen cross-links compared with age- and gender-matched controls. This change could result in a reduction of the material strength of the bone trabeculae and explain why the individuals with osteoporosis had fractures even though they had a similar amount of trabecular bone as the healthy controls [4].
Prolidase (E.C. 4.3.13.9.) is a cytosolic enzyme that is necessary for specific splitting of imidodipeptides at C-terminal proline or hydroxyproline [3]. The final step of collagen degradation is catalysed by prolidase and the lack of this enzyme activity can severely impede the efficient recycling of proline for collagen resynthesis [5]. The relationship between collagen and prolidase activity was observed during fibrotic processes, where an increase in prolidase activity was accompanied by increase in tissue collagen deposition [7]. Moreover, the link between collagen production and prolidase activity has been found in cultured human skin fibroblasts treated with anti-inflammatory drugs, anthracyclines during experimental aging of these cells, fibroblasts chemotaxis and cell surface integrin receptor ligation [5, 9]. In view of the above facts, prolidase activity may be an important factor in the regulation of collagen biosynthesis. Some studies have also been made about the serum prolidase activity and collagen turnover [3, 11, 12]. Namiduru et al. observed positive correlation between cord blood prolidase activity and birth weight as a result of increased collagen metabolism. It has also been suggested that its serum activity was elevated in metabolic bone diseases [3, 12], however; its activity in postmenopausal osteoporotic women has not been studied. Our aim of the study was (1) to investigate serum prolidase activity in postmenopausal osteoporosis (2) its correlation with other bone turnover markers and BMD.
Materials and methods
Subjects
Three populations were studied. The first consisted of 45 postmenopausal osteoporotic women aged 45–70 years (mean 55.68±5.58 years). The second group comprised 55 postmenopausal nonosteoporotic women aged 43–69 years (mean 55.21±6.21 years). The women had natural menopause and no menstrual bleeding for at least 1 year since their last menstruation in the first two groups. The third group consisted of 38 premenopausal nonosteoporotic women aged 30–54 years (mean 41.42±6.19 years) who had regular menstrual cycles. The ethical committee of Gaziantep University Hospital approved this study, and a written informed consent was obtained from each patient.
None of the women had a history of metabolic bone disease or previous fracture, and none were taking any medication known to affect bone metabolism. None of the selected postmenopausal women had been treated with hormone therapy, bisphosphonates, or calcitonin before entry into the study. They had normal hepatorenal function and were free from endocrine disturbances, diabetes mellitus and thyroid disorders. There were no significant abnormalities in urinary calcium excretion, no history of hypercalcuria or urolithiasis in either group.
Measurement of bone markers
A blood sample and a spot urine sample were collected at 9:00 and 11:00 a.m. after an overnight fast and between days 2 and 6 of the menstrual cycle in premenopausal group. There were no strict controls over sleeping hours, diet or physical activities. All subjects continued their usual routine of diurnal activity and nocturnal rest.
Serum total alkaline phosphatase (ALP) was determined by spectrophotometric (using p-nitrophenyl phosphate) method (Abbott, Aeroset, USA). Both the intra and interassay coefficients of variation (CV) were less than 3.9%. Serum C-terminal telopeptide of type I collagen (C-telopeptide) was measured by chemiluminescent immunometric assay (ECLIA Elecsys 170, Roche, Germany). The intra and interassay CV were 2.5 and 4.5%. Urinary deoxypyridinoline (Dpd) was determined by chemiluminescent immunometric assay (Immulite 2000, USA). The intra and interassay CV were 2.5 and 4.5%. Urinary creatinine was measured by spectrophotometric Jaffe’s method (alkaline picrate without deprotein, Abbott), the intra and interassay CV were less than 2 and 4%, respectively. Osteocalcin (OC) was assayed by chemiluminescent immunometric assay (Immulite 2000). The intraassay variation was 2.35% and interassay variation was 2.55%.
Prolidase assay
Serum was diluted 40-fold with 2.5 mmol/l Mn2+, 40 mmol/l Trizma HCl buffer (pH 8.0) and preincubated at 37°C for 2 h. The reaction mixture containing 30 mmol/l gly-pro, 40 mmol/l trizma HCl buffer (pH 8.0) and 100 μl of preincubation serum in 1 ml was incubated at 37°C for 30 min. Adding 0.5 ml of 20% trichloroacetic acid solution then stopped the incubation reaction. The supernatant was used for measurement of proline by the method proposed by Myara which is a modification of Chinard’s method [10]. All reagents were of analytical grade and obtained from Sigma (St. Louis, USA) and Merck (Darmstadt, Germany). Intra and interassay precision performances of the assay were determined from a serum pool on ten replicates in a single run and in ten different runs, respectively, yielded CV of 3.8 and 9.0%.
Bone density measurements
Bone mineral density (BMD) of lumbar spine and femoral neck were measured by Hologic QDR 4500 W (Hologic Inc., Bedford, MA, USA) fan beam DXA scanner. The within subject coefficient of variations were both 1% for lumbar spine and the femoral neck.
Statistical evaluation
Values shown in the text and tables are mean ± SD. Analysis of covariance was used to measure age, bone turnover markers, BMD and prolidase levels followed by Scheffe F test. Pearson’s correlation coefficient was estimated to quantify the strength of the association between prolidase, bone turnover markers and BMD. Significance was defined as P<0.05. The entire statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) 11.0 for Windows.
Results
Table 1 showed the patient characteristics, mean ± SD of each bone turnover marker, BMD and prolidase levels between the three groups. There were no significant differences in terms of age and time since menopause between postmenopausal osteoporotic and nonosteoporotic group, but the mean value of age in premenopausal nonosteoporotic women was significantly lower than other groups. There was also no significant difference in mean value of body mass index (BMI) between the groups.
Total ALP was not significantly higher in postmenopausal osteoporotic women compared with postmenopausal nonosteoporotic group, but OC, C-telopeptide and urinary Dpd levels in postmenopausal osteoporotic women were significantly higher than in postmenopausal nonosteoporotic women. In addition, total ALP, OC, C-telopeptide and urinary Dpd levels in postmenopausal osteoporotic and postmenopausal nonosteoporotic group were significantly higher than in premenopausal nonosteoporotic group. Although prolidase activity was lower in postmenopausal osteoporotic women compared with postmenopausal nonosteoporotic and premenopausal nonosteoporotic group, there was no significant difference in prolidase levels between the groups (P>0.05).
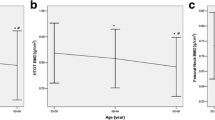
There was also no correlation between serum prolidase levels and bone turnover markers in postmenopausal osteoporotic women (Fig. 1). Moreover, no significant correlation exists between serum prolidase and LS BMD and FNBMD in postmenopausal osteoporotic patients (r=0.63, P>0.05 and r=0.28, P>0.05, respectively).
Discussion
Prolidase is a highly specific peptidase. It is the only enzyme known to catalyse hydrolysis of compounds in which the sensitive peptide bond involves the imino nitrogen of proline or hydroxyproline and provides the conservation of proline [11]. The conformational restrictions imposed by proline in a peptide chain appear to imply important structural or biological functions. This enzyme plays an important functional role and is expressed in all human tissues and cells. Hereditary prolidase deficiency, the multisystemic disorder is characterized by a wide spectrum of clinical manifestations including skin ulcers, mental retardation and susceptibility to infections.
This is the first report about the serum prolidase concentrations and its correlation with bone turnover markers and BMD in postmenopausal osteoporotic women. In the present study, this enzyme activity in plasma was found to be slightly decreased in postmenopausal osteoporosis, but it was not statistically different from other groups without osteoporosis. Moreover, no correlation was observed between this enzyme activity and markers of bone turnover or BMD in postmenopausal osteoporosis.
There are few studies about the relationship between serum prolidase concentrations and bone metabolism [3, 5, 8, 12]. Oner et al. [12] studied the effect of low dose oral theophylline therapy on some bone turnover markers and serum prolidase activity in mild asthmatics. Since theophylline has been known to increase bone turnover, they observed elevated levels of total ALP, OC, urinary hydroxyproline as well as serum prolidase activity. Although no correlations were found between theophylline concentration and bone turnover markers, serum prolidase activity was positively correlated with blood theophylline levels [12].
Erbagci et al. [3] found an association between serum prolidase concentration and bone turnover in diabetic patients. Although, osteoporosis related to type 2 diabetes mellitus was not fully acknowledged, it has been thought that long-term metabolic consequences of diabetes would accelerate bone loss and relative deficiency of insulin and/or insulin like growth factors might have implications on collagen metabolism [3, 5, 6]. However, Myara et al. [10] reported that plasma prolidase activity was not elevated in bone metastasis and primary bone cancers. They also emphasized that its plasma level was also unrelated to age and sex.
Osteogenesis imperfecta (OI) is an autosomal dominant disorder associated with defects in the synthesis or structure of type I collagen, the major protein of bone and skin and the molecular defects of type I collagen in fibroblasts from the proband with OI has been shown to be accompanied by a decrease in prolidase activity in these cells [5].
Serum prolidase activity and its relation with some biochemical markers of bone metabolism were studied in metastatic and nonmetastatic breast cancer (BC) patients. It has been observed that serum prolidase levels were significantly higher in BC, but there was no difference between the patients who had bone metastasis or not. They reported that prolidase activity might be increased as a result of high estrogenic state in BC patients since estrogens have been known to stimulate collagen metabolism [1, 14, 15] and this enzyme activity had not been affected by increased bone turnover [8].
In conclusion, we observed that there was no difference in serum prolidase activity in postmenopausal osteoporotic women compared with postmenopausal nonosteoporotic and premenopausal nonosteoporotic controls. We think that prolidase activity might be balanced between two opposite forces, has been suggested to increase as a result of collagen turnover in osteoporosis, and might be negatively affected by lack of estrogen in postmenopausal osteoporosis. Therefore, few studies have demonstrated that collagen synthesis and prolidase activity were stimulated by estrogen in cultured cells [16, 17]. It has also been reported that collagen content in various organs like skin and bone decreases [15] in menopause with loss of estrogen and is known to be reversed by hormone replacement therapy [1, 14]. We think that more studies with larger groups are needed to investigate serum prolidase activity and its relation with bone metabolism in the future.
References
Brincat MP (2000) Hormone replacement therapy and the skin. Maturitas 35:107–117
Consensus Development Statement (1997) Who are candidates for prevention and treatment for osteoporosis? Osteoporos Int 7:1–6
Erbagci AB, Araz M, Erbagci A, Tarakcioglu M, Namiduru ES (2002) Serum prolidase activity as a marker of osteoporosis in type 2 diabetes mellitus. Clin Biochem 35:263–268
Felsenberg D, Boonen S (2005) The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 27:1–11
Gualicka A, Wolczynski S, Anchim T, Surazynski A, Lesniewicz R, Palka J (2001) Defects of type I procollagen metabolism correlated with decrease of prolidase activity in a case of lethal osteogenesis imperfecta. Eur J Biochem 268:2172–2178
Kao WH, Kammerer CM, Schneider JL, Bauer RL, Mitchell BD (2003) Type 2 diabetes is associated with increased bone mineral density in Mexican-American women. Arch Med Res 34:399–406
Karna E, Miltyk W, Wolczynski S, Palka JA (2001) The potential mechanism for glutamine-induced collagen biosynthesis in cultured human skin fibroblasts. Comp Biochem Physiol 130:23–32
Kir ZO, Oner P, Iyidogan YO, Turkmen S, Kocak H, Koser M, Kucucuk SO (2003) Serum prolidase I activity and some bone metabolic markers in patients with breast cancer: in relation to menopausal status. Clin Biochem 36:289–294
Muszynska A, Wolczynski S, Palka J (2001) The mechanism for anthracycline-induced inhibition of collagen biosynthesis. Eur J Pharmacol 411:17–25
Myara I, Myara A, Mangeot M, Fabre M, Charpentier C, Lemonnier A (1984) Plasma prolidase activity: a possible index of collagen catabolism in chronic liver disease. Clin Chem 302:211–215
Namiduru ES, Ozdemir Y, Kutlar I, Ersoy U (2001) A study of prolidase in mothers, their newborn babies and in non-pregnant controls. Arch Gynecol Obstet 265:73–75
Oner P, Gurdol F, Oner-Iyidon Y, Kolanci C, Buyukozturk S (1999) Evaluation of the effect of low-dose oral theophylline therapy on some bone turnover markers and serum prolidase I activity in mild asthmatics. Pharmacol Res 40:189–193
Oxlund H, Mosekilde L, Ortoft G (1996) Reduced concentration of collagen reducible cross links in human trabecular bone with respect to age and osteoporosis. Bone 19:479–484
Sauerbronn AV, Fonseca AM, Bagnoli VR, Saldiva PH, Pinotti JA (2000) The effects of systemic hormonal replacement therapy on the skin of postmenopausal women. Int J Gynaecol Obstet 68:35–41
Sumino H, Ichikawa S, Abe M, Endo Y, Nakajima Y, Minegishi T, Ishikawa O, Kurabayashi M (2004) Effects of aging and postmenopausal hypoestrogenism on skin elasticity and bone mineral density in Japanese women. Endocr J 51:159–164
Surazynski A, Jarzabek K, Haczynski J, Laudanski P, Palka J, Wolczynski S (2003) Differential effects of estradiol and raloxifene on collagen biosynthesis in cultured human skin fibroblasts. Int J Mol Med 12:803–809
Wolczynski S, Surazynski A, Swiatecka J, Palka J (2001) Estrogenic and antiestrogenic effects of raloxifene on collagen metabolism in breast cancer MCF-7 cells. Gynecol Endocrinol 15:225–233
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verit, F.F., Geyikli, I., Yazgan, P. et al. Correlations of serum prolidase activity between bone turnover markers and mineral density in postmenopausal osteoporosis. Arch Gynecol Obstet 274, 133–137 (2006). https://doi.org/10.1007/s00404-006-0148-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-006-0148-9